
����Ŀ����ҵ�Ϸ�������Ϊ���ȼ���е���������1886�귨����ѧ��H��M0issanͨ�������⻯��(KHF2)�ķ�������ˮ��Һ��һ���Ƶ÷�������֪��KF+HF=KHF2���Ʊ������ĵ��װ����ͼ��ʾ������˵���������
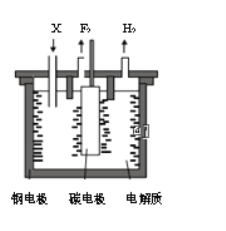
A. �ֵ缫���Դ�ĸ�������
B. �������費�ϲ����X��KF
C. �������������ұ������
D. ���⻯���ڷ������п��Ե���
���𰸡�B
��������
������ڵķ��⻯�أ�KHF2���ͷ����⣨HF��������Ʊ������ʣ�������������ʧ�������ɷ�����������KHF2�õ����������������缫��ӦΪ��2HF2-+2e-�TH2��+4F-��A. ����ͼʾ���ֵ缫�ϲ���������������ԭ��Ӧ���ֵ缫Ϊ���������Դ�ĸ�����������A��ȷ��B. �������⣬�������л�����ķ��⻯�أ�KHF2�����費�ϲ����X�Ƿ��⻯�أ�KHF2������B����C. ����Ʒ�ʱ��Ϊ�˷�ֹ�����������������ҷ�Ӧ��������ը���������������ұ����������C��ȷ��D. �����������������⻯���ڷ������п��Ե����HF2-����D��ȷ����ѡB��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M��R��Ϊ����Ԫ�أ���֪M��һ��ԭ��ʧȥ2�����ӣ�R��һ��ԭ�ӵõ�1�����Ӻ��γ�ϡ������Ԫ�صĵ��Ӳ�ṹ�����й���M��R�γɵĻ������������ȷ����(����)
A. M��R�����γ�MR2�����ӻ�����
B. ��MR2��M�����Ӱ뾶��R�����Ӱ뾶��
C. �γɵ��������ӷֱ���M����R2��
D. MR2�ĵ���ʽΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ֳƼ��ѣ����DME���۵�Ϊ-141.5�棬�е�Ϊ-24.9�档��������Һ��ʯ����(LPG)���ƣ�����Ϊ��21���͵����ȼ�ϡ����ɺϳ���(CO��H2)�Ʊ������ѵķ�Ӧԭ�����£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1
CH3OH(g) ��H1= -90.0 kJ��mol-1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
�ش��������⣺
��1����Ӧ��Ϊ��___________(����ӡ����١�)�ķ�Ӧ��__________ (����¡����¡�)�����Է����С�
��2��д���ɺϳ���(CO��H2) ֱ���Ʊ������ѵ��Ȼ�ѧ����ʽ��__________________________________��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ�١��ڣ�5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1����H2��ʾ��Ӧ�ٵ�������______________����Ӧ�ڵ�ƽ�ⳣ��K=________________��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱ��Ӧ�ڵ�v��__ v��(����>���� ��< ������=��)��
��4���о����֣��������ͬ�������м������ʵ�����ͬ��CO��H2������Ӧ�١��ڣ��ڲ�ͬ�¶Ⱥ�����������¾�����ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | COת����(%) | CH3OCH3ѡ����(%) |
473 | �� | 10 | 36 |
500 | �� | 12 | 39 |
500 | Cu/ZnO | 20 | 81 |
[��ע]������ѡ���ԣ�ת����CO������CH3OCH3�İٷֱ�
����ͬ�¶��£�ѡ��Cu/ZnO���������ô�����____________ (����)��
A.�ٽ�ƽ�������ƶ� B.��߷�Ӧ���� C.���ͷ�Ӧ�Ļ��
D.�ı䷴Ӧ���ʱ� E.���CO��ƽ��ת����
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬��ԭ����_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������þƾ��Ƽ���ʱ�����ʯ�������ǣ� ��
���ձ� ������ ����ƿ �������� ���Թ� ����ƿ �߱�����
A.�ڢܢ�B.�٢ޢ�C.�ۢܢ�D.�٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������M�Ƕ�����Ȳ��Һ�����ϵ�һ�֣���Ķ�����Ȳ�������![]() ���Ի�Ϊͬϵ��ĵ�ȡ��������A��GΪԭ�Ϻϳ�M��һ��·��(���ַ�Ӧ������ȥ)���£�
���Ի�Ϊͬϵ��ĵ�ȡ��������A��GΪԭ�Ϻϳ�M��һ��·��(���ַ�Ӧ������ȥ)���£�
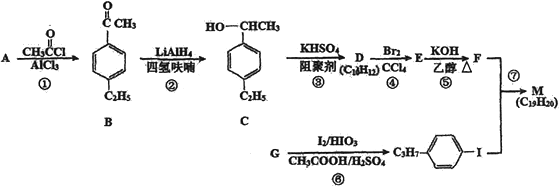
�ش��������⣺
��1��A�Ľṹ��ʽΪ________________�� B�еĹ�����������____________________��
��2���ڵķ�Ӧ������_______________��F������Ϊ____________________��
��3��д����C����B�Ļ�ѧ����ʽ______________________________________��
��4����Ӧ�ݵĻ�ѧ����ʽ��______________________________________��
��5��B��ͬ���칹�������㱽����������ȡ�������ܷ���������Ӧ����________��(���������칹)��B����һ��ͬ���칹���У����������һ��������˴Ź�������Ϊ4��壬�ҷ������Ϊ2:2:1:1����ͬ���칹��Ľṹ��ʽΪ__________________��
��6�����������ϳ�·�ߣ����һ���ɱ���ϩ�ͼױ�Ϊ��ʼԭ���Ʊ�![]() �ĺϳ�___��
�ĺϳ�___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��t��ʱ��AgX(X=Cl��Br)���ܶȻ���c(Ag+)��c(X-)�����ϵ����ͼ��ʾ������A�߱�ʾAgCl��B�߱�ʾAgBr����֪p(Ag+)=��lgc(Ag+)��p(X-)=��lgc(X-)������˵����ȷ���ǣ� ��

A. c��ɱ�ʾAgCl�IJ�������Һ
B. b���AgCl��Һ����AgNO3������Ա��a��
C. t��ʱ��AgCl(s)+Br-(aq)![]() AgBr(s)+Cl-(aq)ƽ�ⳣ��K=10-4
AgBr(s)+Cl-(aq)ƽ�ⳣ��K=10-4
D. t��ʱ��ȡa���AgCl��Һ��b��AgBr��Һ�������ϣ�������AgBr����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У��������ͼʾ������һ����������һ��ת���������( )
��� | X | Y | Z | W |
|
�� | Cu | CuSO4 | Cu(OH)2 | CuO | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Al | AlCl3 | Al(OH)3 | Al2O3 | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A. �ڢۢ�B. �٢ۢ�C. �٢ڢ�D. �٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Fe3O4�ʺ�ɫ�������д���������С���ڴż�¼���ϡ����﹦�ܲ��ϵ������������ҪӦ�ã�̽�����Ʊ�����;�����ش�
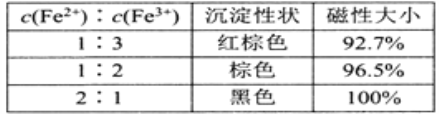
��1����ԭ-�����������û�ԭ��Na2SO3��һ����Fe3+��������Һ�е�![]() Fe3+��ԭ��ʹFe2+��Fe3+�����ʵ�����Ϊ1��2����Ȼ���ڢ�������ϵ�м��백ˮ����Ԫ����ȫ�����γ�����Fe3O4��д���ڹ��̵����ӷ���ʽ��_______������ԭ����Һ��c(Fe2+)��c(Fe3+)=2��1ʱ�����±����ݿ�֪����Ʒ��������ܵ�ԭ����________________��
Fe3+��ԭ��ʹFe2+��Fe3+�����ʵ�����Ϊ1��2����Ȼ���ڢ�������ϵ�м��백ˮ����Ԫ����ȫ�����γ�����Fe3O4��д���ڹ��̵����ӷ���ʽ��_______������ԭ����Һ��c(Fe2+)��c(Fe3+)=2��1ʱ�����±����ݿ�֪����Ʒ��������ܵ�ԭ����________________��
��2���绯ѧ��Ҳ���Ʊ�����Fe3O4�������Ϊ4cm2�IJ����С��(����������)Ϊ�����缫����˿����������Na2SO4��Һ��Ϊ���Һ�����Һ��pHά����10���ң�����50mA������Fe3O4�ĵ缫��ӦΪ__________��
��3����֪��H2O(1)=H2(g)+![]() O2(g)��H=+285��5kJ��mol-1����̫����Ϊ��Դ�ֽ�Fe3O4�������Ȼ�ѧ����������ѭ���ֽ�ˮ��H2�Ĺ������£��������¹���I���Ȼ�ѧ����ʽ��
O2(g)��H=+285��5kJ��mol-1����̫����Ϊ��Դ�ֽ�Fe3O4�������Ȼ�ѧ����������ѭ���ֽ�ˮ��H2�Ĺ������£��������¹���I���Ȼ�ѧ����ʽ��
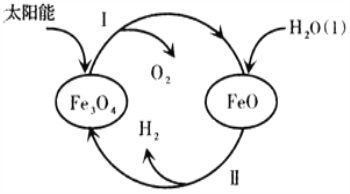
����I��____________________________________________________��
����II��3FeO(s)+H2O(1)===H2(g)+Fe3O4(s) ��H=+128��9kJ��mol-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�
![]()
(1)д��A��B��C��Ԫ������________��________��________��
(2)C��Ԫ�����ڱ��е�λ����____________________��
(3)B��ԭ�ӽṹʾ��ͼΪ________________��C���⻯����B���⻯����ȶ���ǿ��˳��Ϊ________>________(�ѧʽ)��
(4)�Ƚ�A��C��ԭ�Ӱ뾶A________C��д��A����̬�⻯����A������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com