
�Ӻ�����ͭ����Ͳ��Ľ�����������ȡ����������һ�ֹ������£�
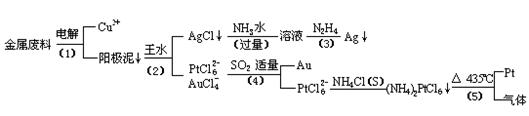
�������Ϲ��ջش��������⣺
���ʱ���Խ�������Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ��д����ⷽ��ʽ��
������
������ ��
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ��������,�ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��
��3��д�����裨4�������ӷ�Ӧ����ʽ��
��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��
Au + 6HNO3��Ũ��= Au(NO3)3 + 3NO2��+ 3H2O
���÷�Ӧ��ƽ�ⳣ����С�����Խ��Ũ���Ἰ������Ӧ������ȴ����������ˮ���Լ�Ҫ����֮��
��
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 2Ag+4NH3+CH2OH��CHOH��4COOH+H2O
2Ag+4NH3+CH2OH��CHOH��4COOH+H2O 2Ag+4NH3+CH2OH��CHOH��4COOH+H2O
2Ag+4NH3+CH2OH��CHOH��4COOH+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
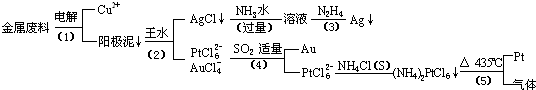
�Ӻ�����ͭ����Ͳ��Ľ�����������ȡ����������һ�ֹ������£�
�������Ϲ��ջش��������⣺
��1�� ���ʱ���Խ�������Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ��д����ⷽ��ʽ��
������
������ ��
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ��������,�ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��
��3��д�����裨4�������ӷ�Ӧ����ʽ��
��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��
Au + 6HNO3��Ũ��= Au(NO3)3 +3NO2��+ 3H2O
���÷�Ӧ��ƽ�ⳣ����С�����Խ��Ũ���Ἰ������Ӧ������ȴ����������ˮ���Լ�Ҫ����֮��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�ϲ��и���12���¿���ѧ�Ծ� ���ͣ������
�Ӻ�����ͭ����Ͳ��Ľ�����������ȡ����������һ�ֹ������£�
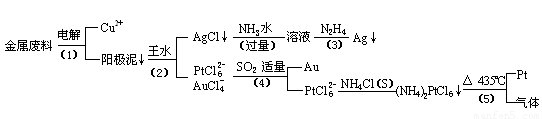
�������Ϲ��ջش��������⣺
��1�� ���ʱ���Խ�������Ϊ��������ͭΪ������CuSO4��ҺΪ���Һ��д����ⷽ��ʽ��
������
������ ��
��2��AgCl���ڰ�ˮ�����õ���Һ���е�һ��������,�ڼ��������£�Ҳ���������ǽ��仹ԭΪ����д���÷�Ӧ�����ӷ���ʽ��
��
��3��д�����裨4�������ӷ�Ӧ����ʽ��
��
��4�����Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��
Au + 6HNO3��Ũ��= Au(NO3)3 + 3NO2��+ 3H2O
���÷�Ӧ��ƽ�ⳣ����С�����Խ��Ũ���Ἰ������Ӧ������ȴ����������ˮ���Լ�Ҫ����֮��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com