
| A��W2Y��Һ������ |
| B��W��Z�γɵĻ������в��������Ӽ� |
| C��1molԪ��Z�ĵ�����������W������������Ӧ��ˮ�������Һ��Ӧ��ת��1mol���� |
| D��W��X�ĵ���ͨ���ǵ����������õ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
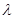 ����Ƶ��v�Ĺ�ϵΪ
����Ƶ��v�Ĺ�ϵΪ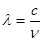 �����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е���������������kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ�� ����������������
�����й���c=3��108m��s-1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ����Ϊ300 nm�������Ĺ��������е���������������kJ��mol��1��˵�����峤ʱ������������Ƥ���Ƿ�����˺���ԭ�� ����������������| ���ۼ� | C��C | C��N | C��S |
| ����/kJ��mol��1 | 347 | 305 | 259 |
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����
���� ����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)��
����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)��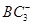 ������Bԭ�ӹ�����ӻ�����Ϊ__________��
������Bԭ�ӹ�����ӻ�����Ϊ__________��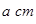 ���þ�����ܶ�Ϊ________
���þ�����ܶ�Ϊ________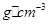 g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��
g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��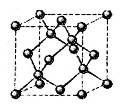
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| �� | �� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/10 -10m | 0.37 | 1.86 | 0.74 | 1.43 | 0.77 | 1.10 | 0.99 | 1.52 | 0.75 | 0.71 |
| ���̬ | +1 | +1 | �� | +3 | +4 | +5 | +7 | +1 | +5 | �� |
| ��ͼ�̬ | -1 | �� | -2 | �� | -4 | -3 | -1 | �� | -3 | -1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | R | | |
| T | Q | | W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��X��Y��Z�ĺ����������������ǿ |
| B��WԪ���γɵĵ��������ӻ�ԭ��ǿ��X |
| C��W��X��Y��ԭ�Ӱ뾶�������� |
| D��W����Ԫ���γɵĻ������п��ܺ��зǼ��Լ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com