
ЁОЬтФПЁПдкЪвЮТЯТЃЌЯТСаЮхжжШмвКЃК
Ђй0.1mol/L NH4Cl Ђк0.1mol/L CH3COONH4 Ђл 0.1mol/L NH4HSO4
Ђм0.1mol/L NH3H2OКЭ0.1mol/L NH4ClЛьКЯвК Ђн0.1mol/L NH3H2O
ЧыИљОнвЊЧѓЬюаДЯТСаПеАзЃК
ЃЈ1ЃЉШмвКЂйГЪ_________адЃЈЬюЁАЫсЁБЁЂЁАМюЁБЛђЁАжаЁБЃЉЃЌЦфдвђЪЧ_____________ЃЈгУРызгЗНГЬЪНБэЪОЃЉЁЃ
ЃЈ2ЃЉдкЩЯЪіЮхжжШмвКжаЃЌpHзюаЁЕФЪЧ_____ЃЛcЃЈNH4+ЃЉзюаЁЕФЪЧ____ЃЈЬюађКХЃЉЁЃ
ЃЈ3ЃЉдкШмвКЂмжаЃЌ_____________РызгЕФХЈЖШЮЊ0.1mol/LЃЛNH3H2OКЭ________РызгЕФЮяжЪЕФСПХЈЖШжЎКЭЮЊ0.2 mol/LЁЃ
ЃЈ4ЃЉЪвЮТЯТЃЌВтЕУШмвКЂкЕФpH=7ЃЌдђCH3COOЃгыNH4+ХЈЖШЕФДѓаЁЙиЯЕЪЧЃКcЃЈCH3COOЃЃЉ_____________cЃЈNH4+ЃЉЃЈЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБЃЉЁЃ
ЁОД№АИЁПЫс NH4++H2O![]() H++NH3H2O Ђл Ђн Cl- NH4+ =
H++NH3H2O Ђл Ђн Cl- NH4+ =
ЁОНтЮіЁП
бЮРрЫЎНтвЛАуЪЧЮЂШѕЕФЃЌИљОнгАЯьбЮРрФмЫЎНтРызгЕФвђЫиЃЌНсКЯШѕЕчНтжЪЕФЕчРыЃЌХаЖЯШмвКЕФЫсМюадвдМАЯрЙиРызгХЈЖШЕФДѓаЁЃЌHSO4-ЕчРыГіЕФH+вжжЦNH4+ЕФЫЎНтЃЛЕШЮяжЪЕФСПХЈЖШЕФNH3H2OКЭ0.1mol/L NH4ClЛьКЯвКЃЌNH3H2OЕФЕчРыДѓгкNH4+ЫЎНтЃЌШмвКГЪМюадЃЛNH4+ЁЂCH3COO-ЖМЫЎНтЃЌВЂЧвЫЎНтЯрЛЅДйНјГЬЖШЯрЭЌЁЃ
ЃЈ1ЃЉNH4ClЪЧЧПМюШѕЫсбЮЃЌNH4+ЫЎНтЯдЫсадЃЛNH4+ЫЎНтЗДгІЕФРызгЗНГЬЪНNH4++H2O![]() H++NH3H2OЃЛ
H++NH3H2OЃЛ
ЃЈ2ЃЉЫсШмвКжаЧтРызгХЈЖШдНДѓЃЌШмвКpHдНаЁЃЌЂй0.1mol/L NH4ClЃЌNH4+ЫЎНтЯдЫсадЃЌCl-ВЛЫЎНтЃЛЂкCH3COONH4ЪЧШѕЫсШѕМюбЮЃЌ0.1mol/L CH3COONH4ЃЌNH4+ЁЂCH3COO-ЖМЫЎНтЃЌВЂЧвЫЎНтЯрЛЅДйНјГЬЖШЯрЭЌЃЌШмвКГЪжаадЃЛЂл 0.1mol/L NH4HSO4ЃЌHSO4- = H++SO42-ЃЌHSO4-ЕчРыГіЕФH+вжжЦNH4+ЕФЫЎНтЃЛЂм0.1mol/L NH3H2OКЭ0.1mol/L NH4ClЛьКЯвКЃЌNH3H2OЕФЕчРыДѓгкNH4+ЫЎНтЃЌШмвКГЪМюадЃЌШмвКжаNH4+ЫЎНтЕФХЈЖШДѓгк0.1mol/LЃЛЂн0.1mol/L NH3H2OЃЌNH3H2OЪЧШѕЕчНтжЪЃЌNH3H2O![]() NH4++OH-ЃЌ NH3H2OЮЂШѕЕчРыЩњГЩNH4+ЃЛОЩЯЪіЗжЮіПЩжЊШмвКpHзюаЁЕФЪЧЂл 0.1mol/L NH4HSO4ЃЛc(NH4+)зюаЁЕФЪЧЂн0.1mol/L NH3H2OЃЛ
NH4++OH-ЃЌ NH3H2OЮЂШѕЕчРыЩњГЩNH4+ЃЛОЩЯЪіЗжЮіПЩжЊШмвКpHзюаЁЕФЪЧЂл 0.1mol/L NH4HSO4ЃЛc(NH4+)зюаЁЕФЪЧЂн0.1mol/L NH3H2OЃЛ
ЃЈ3ЃЉЂм0.1mol/L NH3H2OКЭ0.1mol/L NH4ClЛьКЯвКЃЌТШРызгЕФЮяжЪЕФСПВЛБфЁЂХЈЖШВЛБфЃЌТШРызгЕФХЈЖШЮЊ0.1mol/LЃЛNH3H2OЕФЕчРыДѓгкNH4+ЫЎНтЃЌШмвКГЪМюадЃЌгЩЮяСЯЪиКуПЩжЊЃЌc(NH3H2O)+c(NH4+)=0.2 mol/LЃЛ
ЃЈ4ЃЉЪвЮТЯТЃЌВтЕУШмвКЂкЕФpH=7ЃЌNH4+ЁЂCH3COO-ЖМЫЎНтЃЌВЂЧвЫЎНтЯрЛЅДйНјГЬЖШЯрЭЌЃЌc(CH3COO-)=c(NH4+)ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПCЁЂNЁЂPЁЂSЁЂFeЁЂCuЪЧгыШЫРрЩњВњЁЂЩњЛюЯЂЯЂЯрЙиЕФЛЏбЇдЊЫиЃЌРћгУЫљбЇжЊЪЖЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌCuдзгКЫЭтЕчзгХХВМЪНЮЊ___ЃЌдђИпЮТЯТЕФЮШЖЈадCuO___Cu2OЃЈЬюЁАЃОЁБЛђЁАЃМЁБЃЉЃЛ
ЃЈ2ЃЉОбаОПCuЕФФГжжЛЏКЯЮяФмДпЛЏбѕЛЏCO(NH2)2ЃЌЦфжаCЁЂNЕФдгЛЏЗНЪНЗжБ№ЮЊ___ЃЌ___ЃЛ
ЃЈ3ЃЉSiЁЂPЁЂSдЊЫиЕФЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЪЧ___ЃЛ
ЃЈ4ЃЉOF2ЕФПеМфЙЙаЭЪЧ___ЃЌЗжзгМЋадЃКH2O___OF2ЃЈЬюЁАЃОЁБЛђЁАЃМЁБЃЉЃЌРэгЩЪЧ___ЃЛ
ЃЈ5ЃЉ[Fe(CN)6]3-жаХфЮЛМќКЭІаМќЕФИіЪ§жЎБШЮЊ___ЃЛ
ЃЈ6ЃЉСкєЧЛљБНМзШЉЕФЗаЕуЕЭгкЖдєЧЛљБНМзШЉЕФЗаЕуЃЌдвђЪЧ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊСЫХфжЦ![]() ЕФХЈЖШгыCl-ЕФХЈЖШБШЮЊ1:1ЕФШмвКЃЌПЩдкNH4ClШмвКжаМгШы
ЕФХЈЖШгыCl-ЕФХЈЖШБШЮЊ1:1ЕФШмвКЃЌПЩдкNH4ClШмвКжаМгШы
ЂйЪЪСПЕФHCl ЂкЪЪСПЕФNaCl ЂлЪЪСПЕФАБЫЎ ЂмЪЪСПЕФNaOH
A.ЂйЂкB.ЂлC.ЂлЂмD.Ђм
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊИУЗДгІ4NH3+5O2=4NO+6H2OжаЃЌШєЗДгІЫйТЪЗжБ№гУvЃЈNH3ЃЉЁЂvЃЈO2ЃЉЁЂvЃЈNOЃЉЁЂvЃЈH2OЃЉБэЪОЃЌдђе§ШЗЕФЙиЯЕЪЧ
A.![]() vЃЈNH3ЃЉ=vЃЈO2ЃЉB.
vЃЈNH3ЃЉ=vЃЈO2ЃЉB.![]() vЃЈO2ЃЉ=vЃЈH2OЃЉ
vЃЈO2ЃЉ=vЃЈH2OЃЉ
C.![]() vЃЈNH3ЃЉ=vЃЈH2OЃЉD.
vЃЈNH3ЃЉ=vЃЈH2OЃЉD.![]() vЃЈO2ЃЉ=vЃЈNOЃЉ
vЃЈO2ЃЉ=vЃЈNOЃЉ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаГЃЮТЯТХЈЖШОљЮЊ![]() ЕФЯТСа5жжШмвКЃК
ЕФЯТСа5жжШмвКЃК![]() ШмвК
ШмвК![]() ШмвК
ШмвК![]() ШмвК
ШмвК![]() ШмвК
ШмвК![]() ШмвК
ШмвК
![]() ет5жжШмвКpHгЩДѓЕНаЁЕФЫГађЪЧ________________ЃЌЦфжагЩЫЎЕчРыЕФ
ет5жжШмвКpHгЩДѓЕНаЁЕФЫГађЪЧ________________ЃЌЦфжагЩЫЎЕчРыЕФ![]() ХЈЖШзюаЁЕФЪЧ______ЁЃ
ХЈЖШзюаЁЕФЪЧ______ЁЃ![]() ЬюађКХ
ЬюађКХ![]()
![]() жаИїРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ________________________________________________________ЃЌ
жаИїРызгХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ________________________________________________________ЃЌ![]() ЕФЫЎНтЦНКтГЃЪ§
ЕФЫЎНтЦНКтГЃЪ§![]() _________________ЁЃ
_________________ЁЃ![]() вбжЊЬМЫсЕФЕчРыГЃЪ§
вбжЊЬМЫсЕФЕчРыГЃЪ§![]() ЃЌ
ЃЌ![]()
![]() Яђ
Яђ![]() жаЭЈШыЩйСПАБЦјЃЌДЫЪБ
жаЭЈШыЩйСПАБЦјЃЌДЫЪБ ЕФжЕ__________
ЕФжЕ__________![]() ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ
ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ![]() ЁЃ
ЁЃ
![]() ШєНЋ
ШєНЋ![]() КЭ
КЭ![]() ЛьКЯКѓШмвКЧЁКУГЪжаадЃЌдђЛьКЯЧА
ЛьКЯКѓШмвКЧЁКУГЪжаадЃЌдђЛьКЯЧА![]() ЕФЬхЛ§______
ЕФЬхЛ§______![]() ЕФЬхЛ§
ЕФЬхЛ§![]() ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБ
ЬюЁАДѓгкЁБЁЂЁАаЁгкЁБЛђЁАЕШгкЁБ![]() ЁЃ
ЁЃ
![]() НЋ
НЋ![]() ЕФШмвКЗжБ№еєИЩВЂзЦЩеЃЌзюжеПЩЕУдШмжЪЕФЪЧ____________
ЕФШмвКЗжБ№еєИЩВЂзЦЩеЃЌзюжеПЩЕУдШмжЪЕФЪЧ____________![]() ЬюађКХ
ЬюађКХ![]() ЁЃ
ЁЃ
![]() вЛЖЈСПЕФЯЁ
вЛЖЈСПЕФЯЁ![]() ШмвКгызуСПЕФZnЗДгІЃЌЮЊМѕЛКВњЩњЕФ
ШмвКгызуСПЕФZnЗДгІЃЌЮЊМѕЛКВњЩњЕФ![]() ЫйТЪЕЋгжВЛгАЯь
ЫйТЪЕЋгжВЛгАЯь![]() ЕФзмСПЃЌПЩВЩШЁЕФДыЪЉЪЧ______
ЕФзмСПЃЌПЩВЩШЁЕФДыЪЉЪЧ______![]() ЬюзжФИ
ЬюзжФИ![]() ЁЃ
ЁЃ
A.Мг![]() ЙЬЬх
ЙЬЬх![]() Мг
Мг![]() ЙЬЬх
ЙЬЬх![]() Мг
Мг![]() ШмвК
ШмвК![]() МгАБЫЎ
МгАБЫЎ![]() Мг
Мг![]() ШмвК
ШмвК
![]() ГЃЮТЯТЃЌЯђ
ГЃЮТЯТЃЌЯђ![]() ШмвКжаМгШы
ШмвКжаМгШы![]() ШмвКЃЌПЩЙлВьЕНЕФЯжЯѓЪЧ_______________________________________ЃЌЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ______________________________ЃЌШєНЋЫљЕУаќзЧвКЕФpHжЕЕїећЮЊ4ЃЌдђШмвКжа
ШмвКЃЌПЩЙлВьЕНЕФЯжЯѓЪЧ_______________________________________ЃЌЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ______________________________ЃЌШєНЋЫљЕУаќзЧвКЕФpHжЕЕїећЮЊ4ЃЌдђШмвКжа![]() ЕФШмвКЮЊ_____
ЕФШмвКЮЊ_____![]()
![]() вбжЊГЃЮТЯТ
вбжЊГЃЮТЯТ![]() ЁЃ
ЁЃ
![]() ЕШЬхЛ§ЁЂЕШХЈЖШЕФЧтбѕЛЏФЦгыДзЫсЛьКЯКѓШмвКГЪ ____________ адЃЌШмвКжа
ЕШЬхЛ§ЁЂЕШХЈЖШЕФЧтбѕЛЏФЦгыДзЫсЛьКЯКѓШмвКГЪ ____________ адЃЌШмвКжа![]() __________
__________![]() ЬюЁА
ЬюЁА![]() ЁБЁА
ЁБЁА![]() ЁБЛђЁА
ЁБЛђЁА![]() ЁБ
ЁБ![]() ЃЛ
ЃЛ![]() ЕФЧтбѕЛЏФЦгы
ЕФЧтбѕЛЏФЦгы![]() ЕФДзЫсЕШЬхЛ§ЛьКЯКѓШмвКГЪ__________________адЃЌШмвКжа
ЕФДзЫсЕШЬхЛ§ЛьКЯКѓШмвКГЪ__________________адЃЌШмвКжа![]() ______
______![]() ЬюЁА
ЬюЁА![]() ЁБЁА
ЁБЁА![]() ЁБЛђЁА
ЁБЛђЁА![]() ЁБ
ЁБ![]() ЃЛ
ЃЛ
![]() ГЃЮТЯТЃЌгУ
ГЃЮТЯТЃЌгУ![]() ШмвКЕЮЖЈ
ШмвКЕЮЖЈ![]() ФГвЛдЊЫсHAШмвКЫљЕУЕЮЖЈЧњЯпШчЭМЃЎ
ФГвЛдЊЫсHAШмвКЫљЕУЕЮЖЈЧњЯпШчЭМЃЎ
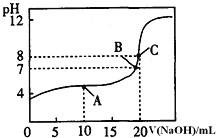
![]() AЁЂBЁЂCШ§ЕуЫљЪОШмвКЕМЕчФмСІзюЧПЕФЪЧ ______ ЕуЖдгІЕФШмвКЃЛ
AЁЂBЁЂCШ§ЕуЫљЪОШмвКЕМЕчФмСІзюЧПЕФЪЧ ______ ЕуЖдгІЕФШмвКЃЛ
![]() ЕуЕФРызгХЈЖШДѓаЁЙиЯЕЪЧ ________________________________ ЃЎ
ЕуЕФРызгХЈЖШДѓаЁЙиЯЕЪЧ ________________________________ ЃЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШ§ТШбѕСз![]() ЙуЗКгУгкХЉвЉЁЂвНвЉЕШЩњВњЁЃЙЄвЕжЦБИШ§ТШбѕСзЕФЙ§ГЬжаЛсВњЩњИБВњЦЗбЧСзЫс
ЙуЗКгУгкХЉвЉЁЂвНвЉЕШЩњВњЁЃЙЄвЕжЦБИШ§ТШбѕСзЕФЙ§ГЬжаЛсВњЩњИБВњЦЗбЧСзЫс![]() ЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЁЃЧыЛиД№ЯТСаЮЪЬтЃК
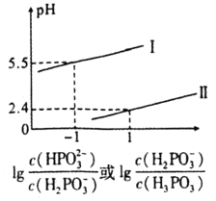
![]() вбжЊбЧСзЫс
вбжЊбЧСзЫс![]() ЮЊЖўдЊШѕЫсЃЌдђ
ЮЊЖўдЊШѕЫсЃЌдђ![]() ШмвКжаЃЌИїРызгХЈЖШЕФДѓаЁЙиЯЕЮЊ________ЁЃ
ШмвКжаЃЌИїРызгХЈЖШЕФДѓаЁЙиЯЕЮЊ________ЁЃ
![]() ГЃЮТЯТЃЌНЋNaOHШмвКЕЮМгЕНбЧСзЫс
ГЃЮТЯТЃЌНЋNaOHШмвКЕЮМгЕНбЧСзЫс![]() ШмвКжаЃЌЛьКЯШмвКpHгыРызгХЈЖШБфЛЏЕФЙиЯЕШчЭМЫљЪОЁЃдђБэЪО
ШмвКжаЃЌЛьКЯШмвКpHгыРызгХЈЖШБфЛЏЕФЙиЯЕШчЭМЫљЪОЁЃдђБэЪО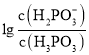 ЕФЪЧЧњЯп________
ЕФЪЧЧњЯп________![]() ЬюЁАЂёЁБЛђЁАЂђЁБ
ЬюЁАЂёЁБЛђЁАЂђЁБ![]() ЃЌбЧСзЫс
ЃЌбЧСзЫс![]() ЕФ
ЕФ![]() ________ЃЌЗДгІ
________ЃЌЗДгІ![]() ЕФЦНКтГЃЪ§жЕЪЧ________ЁЃ
ЕФЦНКтГЃЪ§жЕЪЧ________ЁЃ
![]() ЙЄвЕЩЯЩњВњШ§ТШбѕСзЕФЭЌЪБЛсВњЩњКЌСзЗЯЫЎ
ЙЄвЕЩЯЩњВњШ§ТШбѕСзЕФЭЌЪБЛсВњЩњКЌСзЗЯЫЎ![]() жївЊГЩЗжЮЊ
жївЊГЩЗжЮЊ![]() ЁЂ
ЁЂ![]() ЁЃЯђЗЯЫЎжаЯШМгШыЪЪСПЦЏАзЗлЃЌдйМгШыЩњЪЏЛвЕїНкpHЃЌНЋСздЊЫизЊЛЏЮЊСзЫсЕФИЦбЮГСЕэВЂЛиЪеЁЃШєДІРэКѓЕФЗЯЫЎжа
ЁЃЯђЗЯЫЎжаЯШМгШыЪЪСПЦЏАзЗлЃЌдйМгШыЩњЪЏЛвЕїНкpHЃЌНЋСздЊЫизЊЛЏЮЊСзЫсЕФИЦбЮГСЕэВЂЛиЪеЁЃШєДІРэКѓЕФЗЯЫЎжа![]() ЃЌдђШмвКжа
ЃЌдђШмвКжа![]() ________
________![]() ЁЃ
ЁЃ![]() вбжЊ
вбжЊ![]() ЃЉ
ЃЉ![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОзАжУЃЌЕчСїБэжИеыЗЂЩњЦЋзЊЃЌЭЌЪБAМЋж№НЅБфДжЃЌBМЋж№НЅБфЯИЃЌCЮЊЕчНтжЪШмвКЃЌдђAЁЂBЁЂCгІЪЧЯТСаИїзщжаЕФ(ЁЁЁЁ)

A. AЪЧZnЃЌBЪЧCuЃЌCЮЊЯЁСђЫс
B. AЪЧCuЃЌBЪЧZnЃЌCЮЊЯЁСђЫс
C. AЪЧFeЃЌBЪЧAgЃЌCЮЊЯЁAgNO3ШмвК
D. AЪЧAgЃЌBЪЧFeЃЌCЮЊЯЁAgNO3ШмвК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЏбЇгыЩњВњЁЂЩњЛюУмЧаЯрЙиЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ ( )
A.ЪЙгУвНгУОЦОЋЩБОњЯћЖОЕФЙ§ГЬжажЛЗЂЩњСЫЮяРэБфЛЏ
B.вНгУПкежЮоЗФВМЕФдВФСЯГЩЗжжЎвЛЪЧОлБћЯЉЃЌЦфНсЙЙМђЪНЮЊ![]()
C.впУчвЛАугІРфВиДцЗХЃЌвдБмУтЕААзжЪБфад
D.вНгУЗРЛЄЗўЕФКЫаФВФСЯЪЧЮЂПзОлЫФЗњввЯЉБЁФЄЃЌЦфЕЅЬхЫФЗњввЯЉЪєгкТБДњЬў
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвьЧиЦЄрЄОпгаПЙжзСіЙІаЇЃЌЧиЦЄЫиОпгаПЙСЁМВИЫОњЙІаЇЃЌЫќУЧдквЛЖЈЬѕМўЯТПЩЗЂЩњШчЭМЫљЪОЕФзЊЛЏЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ( )

A.вьЧиЦЄрЄгыЧиЦЄЫиЛЅЮЊЭЌЯЕЮя
B.ЧиЦЄЫивЛЖЈЬѕМўЯТФмЗЂЩњМгГЩЗДгІЁЂЯћШЅЗДгІКЭШЁДњЗДгІ
C.1 mol ЧиЦЄЫизюЖрПЩгы 3 mol NaOH ЗДгІ
D.УПИівьЧиЦЄрЄЗжзггыЧтЦјЭъШЋМгГЩКѓЕФВњЮяжаКЌга 5 ИіЪжадЬМдзг
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com