
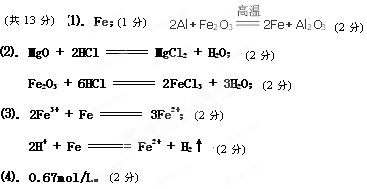
 2Fe��Al2O3
2Fe��Al2O3


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeO | B��Fe2O3 | C��Fe | D��Fe3O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��FeCl2 | B��Fe(OH) 3 | C��H 2SiO4 | D��SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ԣ�Zn2��>Cu2��>Fe3��>Ag�� | B��Fe3���������Դ���Cu2�� |
| C����Һ��Cu2����Fe2�������ʵ�����Ϊ1��2 | D��1 mol Fe�ɻ�ԭ2 mol Fe3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��KSCN��Һ����Һ��ɫ�����Ա仯���Խ���ԭ�� ��
��KSCN��Һ����Һ��ɫ�����Ա仯���Խ���ԭ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��������һ����Cu | B����Һ��һ������Fe2��������һ������Cu2�� |
| C��������һ����Fe | D��������һ����ͭ������һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 _______������ĸ����
_______������ĸ����| ѡ�� | ������ | ������ | �ж� |
| A | ���ǵؿ��к�����ߵ� ����Ԫ�� | ������������ʹ�õĽ������� | ��ԣ���ԣ��� |
| B | ����������ϡ���ᷴӦ �������� | �����������ܻ�ԭ���� ���õ��� | ��ԣ���ԣ��� |
| C | �����ڹ���Ԫ�� | ��������ijЩ��������������� | �������ԣ��� |
| D | �ڿ��������ı������� �����ܵ�����Ĥ | ��������������Ӧ | ��ԣ���ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��5 | B��4 | C��3 | D��2.408��1024 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �м������ϡ���ᣬ�õ�0.448 L(��״����)һ����ɫ����.д����Ӧ�����ӷ���ʽ�� ��������Һ��c(Cu2��)����������mol/L.
�м������ϡ���ᣬ�õ�0.448 L(��״����)һ����ɫ����.д����Ӧ�����ӷ���ʽ�� ��������Һ��c(Cu2��)����������mol/L.�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com