
����Ŀ��I��ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10.0 mL����ƿ�У�����10.0 mL��KI��Һ(����)�������ķ�ӦΪ��Cl2+2KI��2KCl+I2������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ��������0.01mol��L-1 Na2S2O3��Һ��ϴ��Ȼ��װ��0.01mol��L-1 Na2S2O3��Һ��0�̶����ϣ��ų��¶˼����ڵ����ݣ�����Һ����0�̶Ȼ�0�̶���ijһλ�ã����¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+ 2Na2S4O6���Իش������ʴ�
��1������ټ����ָʾ����_______________________________��
��2�������Ӧʹ��________ʽ�ζ��ܡ�
��3���жϴﵽ�ζ��յ��ʵ��������___________________________________��
������4������0.1032 mol/L HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ�����������ʵ������Ӱ�����____________��
A.��ʽ�ζ���δ�ñ�������Һ��ϴ
B.��ƿδ�ô���Һ��ϴ
C.�ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D.�ζ�ʱ����Һ������ƿ��
��5��̼��H2CO3��K1=4.3��10-7��K2=5.6��10-11������H2C2O4 K1=5.9��10-2��K2=6.4��10-5��0.1 mol/L Na2CO3��Һ��pH____________0.1 mol/L Na2C2O4��Һ��pH(ѡ��������������С��������������)��������Ũ�ȵIJ�����Һ��̼����Һ�������ϣ���Һ�и�������Ũ�ȴ�С��˳����ȷ����_____________��
A��c(H+)��c(HC2O4-)��c(HCO3-)��c��CO32-)
B��c(HCO3-)��c(HC2O4-)��c(C2O42-)��c(CO32-)
C��c(H+)��c(HC2O4-)��c(C2O42-)��c(CO32-)
D��c(H2CO3) ��c(HCO3-)��c(HC2O4-)��c(CO32-)
���𰸡�������Һ��������һ�α�Һ����Һ����ɫ�����ɫ�Ұ�����ڲ��ָ�B����AC
��������
(1)��Һ���е��ʵ⣬���������Һ����ɫ�������������Ʒ���������ԭ��Ӧ������Ӧ�յ�ʱ����ɫ��ȥ���ʴ�Ϊ��������Һ��
(2)�����������Һ�Լ��ԣ�Ӧѡ���ʽ�ζ��ܣ��ʴ�Ϊ���
(3)�������۱���ɫ��������Һ����ɫ���淴ӦI2+2Na2S2O3=2NaI+2Na2S4O6���У���Һ��û�е⣬��Һ����ɫΪ��ɫ��˵����Ӧ���յ㣬�жϴﵽ�ζ��յ��ʵ�������ǣ������һ����Һ������ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ���ʴ�Ϊ�������һ����Һ������ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
(4)A����ʽ�ζ���δ�ñ�������Һ��ϴ���������Ũ��ƫС�����V(��)ƫ����c(����)=![]() ��֪���ⶨc(����)ƫ��A����B����ƿδ�ô���Һ��ϴ����V(��)��Ӱ�죬����c(����)=
��֪���ⶨc(����)ƫ��A����B����ƿδ�ô���Һ��ϴ����V(��)��Ӱ�죬����c(����)=![]() ��֪���ⶨc(����)��Ӱ�죬��B��ȷ��C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ�ˣ����V(��)ƫ����c(����)=
��֪���ⶨc(����)��Ӱ�죬��B��ȷ��C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ�ˣ����V(��)ƫ����c(����)=![]() ��֪���ⶨc(����)ƫ��C����D���ζ�ʱ����Һ������ƿ�⣬���V(��)ƫС������c(����)=
��֪���ⶨc(����)ƫ��C����D���ζ�ʱ����Һ������ƿ�⣬���V(��)ƫС������c(����)=![]() ��֪���ⶨc(����)ƫС����D����ѡB��
��֪���ⶨc(����)ƫС����D����ѡB��
(5)����Ķ������볣������̼��Ķ������볣����˵��������������Ա�̼�������ǿ����0.1 mol/L Na2CO3��Һ��̼�����ˮ��̶ȴ���0.1 mol/L Na2C2O4��Һ�в������ˮ��̶ȣ���0.1 mol/L Na2CO3��Һ���Ը�ǿ����0.1mol/LNa2CO3��Һ��pH����0.1mol/LNa2C2O4��Һ��pH�������һ�����������볣��������̼���һ�����볣�������ᡢ̼���һ������Զ���ڶ������룬��һ������Ϊ���������Һ��c (H+)��c (HC2O4-)��c (C2O42-)��c (HCO3-)��c (CO32-)����AC��ȷ��BD���ʴ�Ϊ�����ڣ�AC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾʵ��װ�õ� K �պ�,�����ж���ȷ����
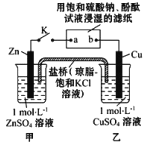
A��Ƭ�̺�׳��� c(SO42-������ B�������� Zn��a��b��Cu ·������
C��Cu �缫�Ϸ�����ԭ��Ӧ D��Ƭ�̺�ɹ۲쵽��ֽ b ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��

(1)ͼ��a��Ҫ���ӵ�Դ��_____(���������)����SO![]() �ŵ�ĵ缫��Ӧ_____________��
�ŵ�ĵ缫��Ӧ_____________��
��.����ͼ��ʾ��װ���У���ͨ��ֱ����5 minʱ��ͭ�缫��������2.16 g���Իش�
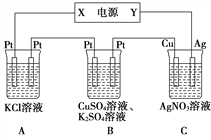
(2)��ҺpH�仯�� B________��C________(�������С�����䡱)��
(3)ͨ��5 minʱ��B�й��ռ�224 mL����(��״��)����Һ���Ϊ200 mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
(4)��A��������KCl��Һ�����Ҳ��200 mL��������Һ��pHΪ_____(����ǰ����Һ����ޱ仯)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Na2O2�ֱ�Ͷ���������ʵ�ˮ��Һ�У����ɰ�ɫ�������ǣ� ��
A. BaCl2 B. K2SO4 C. CuCl2 D. Ca(HCO3)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������G�ĺϳ�·����ͼ��
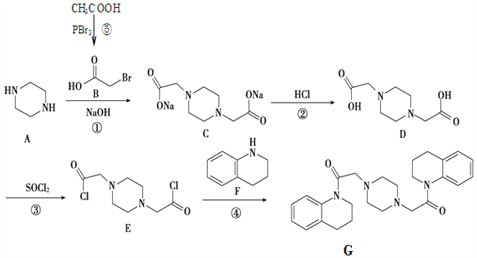
�ش��������⣺
��1������B�й����ŵ�����Ϊ__________________��
��2��д���ٷ�Ӧ���ͣ�__________________��
��3��д������۵ķ�Ӧ����ʽ_____________________________________��
��4�����û�в���ۣ�D��F�ܷ�Ӧ��______��
��5������˵����ȷ����___________��
A ��A�Ƿ����廯����
B ��D���Է���������Ӧ
C ��F ������ԭ�ӹ�ƽ��
D ��G�����뷢���ӳɷ�Ӧ
��6��ͬʱ��������������F��ͬ���칹����______�֣���������ṹ����
�ٺ���C=C��
�ں��б�������ֻ������ȡ����
�ۺ��Щ�NH2
д�����к˴Ź�����ʾ��5����ԭ�ӵ��������ʵĽṹ��ʽ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����г�����ѡ�õ��Լ���������������ȷ��һ����(������Ϊ����)
ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �������� |
A | NaHCO3(Na2CO3) | �������� | �����ᾧ |
B | CO2(CO) | O2 | ��ȼ |
C | Mg(Al) | ����������Һ | ���� |
D | CO2(HCl) | ����������Һ | ϴ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʻ�Ϻ�������NaOH����
A.Na��H2OB.Ca(OH)2��Һ��NaCl��Һ
C.Na2O2��H2OD.Ca(OH)2��Һ��Na2CO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA��ʾ�����ӵ���������ֵ��������������ȷ����
A. ���³�ѹ�£�1.7 g�����к��е�ԭ����ĿΪ0.4NA
B. 50 mL 1 mol��L1 K2SO4��Һ�к��е�K+��ĿΪ0.1NA
C. 5.6 g��������ϡ���ᷴӦת�Ƶĵ�����Ϊ0.3NA
D. ��״���£�4.48 L�������͵����Ļ���ﺬ�еķ�����ĿΪ0.2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ݻ��ɱ���ܱ������г���10 mol N2(g)��10 mol H2(g)����Ӧ��һ�������´ﵽƽ��ʱ��NH3���������Ϊ0.25������[��1������2���ļ��㶼�谴��ʽд������̣�����𰸶�Ҳ������]
��1���������·�ӦN2(g)+3H2(g)![]() 2NH3(g) ��ƽ�ⳣ��________������������£�ÿ1mol������ռ�����ΪVL��
2NH3(g) ��ƽ�ⳣ��________������������£�ÿ1mol������ռ�����ΪVL��
��2��������Ӧ��ƽ��ʱ���ٳ���10 mol��N2�����ݼ��㣬ƽ��Ӧ��ʲô�����ƶ�________
��3��ij�¶��½����õİ������0.1 molL-1����Һ��������Һ��pOH_____��������Һ��ϡ100���������ʱ��ˮ�ĵ����Ϊ________(��֪���¶���Kb(NH3��H2O) =1.0��10-5)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com