
����Ŀ������ijԭ�ӵ�Ħ��������M g /mol����һ����ԭ�ӵ���ʵ������__________g��
��483g Na2SO4��10H2O��������Na+�����ʵ�����________SO42�������ʵ�����_________������H2O���ӵ���Ŀ��_______________����
�DZ�״���£�4 g H2��11.2LO2��1 mLH2O�У�����������������______________��
��ԭ����������_________��������С����___________�������С����__________��
����ͬ״���£���ͬ������ij���������������������Ϊ1�U8����������Ħ������Ϊ______________��
�ɵ�������O2��O3���������ʵ���֮��Ϊ__________���������Ӹ�����Ϊ___________��������ԭ�Ӹ�����Ϊ__________����ͬ״���µ������Ϊ_____________��
���𰸡�![]() 3mol1.5mol9.03��10244gH24gH21mLH2O1mLH2O16g/mol3:23:21:13:2
3mol1.5mol9.03��10244gH24gH21mLH2O1mLH2O16g/mol3:23:21:13:2
��������
��1��һ��ԭ�ӵ���ʵ������![]() g��
g��
��2���ù�ʽn=![]() ����n��Na2SO4��10H2O�������ݻ�ѧʽ����Na+��SO42-��H2O�����ʵ�������һ������H2O����Ŀ��
����n��Na2SO4��10H2O�������ݻ�ѧʽ����Na+��SO42-��H2O�����ʵ�������һ������H2O����Ŀ��
��3���ù�ʽn=![]() ����n��H2����n��H2O�����ù�ʽn=
����n��H2����n��H2O�����ù�ʽn=![]() ����n��O2������Ϸ������ȷ��ԭ�����ʵ������ù�ʽ����������Һ������ԶС������������Ȼ��Ƚϵó�������
����n��O2������Ϸ������ȷ��ԭ�����ʵ������ù�ʽ����������Һ������ԶС������������Ȼ��Ƚϵó�������
��4����ͬ״���£���������֮�ȵ�������������ʵ���֮�ȣ������������Ħ������������������ʵ����ɷ��ȡ�
��5���ù�ʽn=![]() ȷ��O2��O3���ʵ���֮�ȣ�������֮�ȵ��ڷ������ʵ���֮���������ȷ��ԭ����֮������ͬ�������������֮�ȵ�������������ʵ���֮�ȡ�
ȷ��O2��O3���ʵ���֮�ȣ�������֮�ȵ��ڷ������ʵ���֮���������ȷ��ԭ����֮������ͬ�������������֮�ȵ�������������ʵ���֮�ȡ�
��1��ijԭ�ӵ�Ħ��������Mg/mol��1mol��ԭ�ӵ�����ΪMg��1mol��ԭ������ԭ����Ϊ6.02��1023������һ��ԭ�ӵ���ʵ������![]() g��
g��
��2��n��Na2SO4��10H2O��=![]() =1.5mol������Na+���ʵ���n��Na+��=1.5mol��2=3mol��SO42-���ʵ���n��SO42-��=1.5mol��H2O���ʵ���n��H2O��=1.5mol��10=15mol������H2O���ӵ���ĿΪ15mol��6.02��1023mol-1=9.03��1024����
=1.5mol������Na+���ʵ���n��Na+��=1.5mol��2=3mol��SO42-���ʵ���n��SO42-��=1.5mol��H2O���ʵ���n��H2O��=1.5mol��10=15mol������H2O���ӵ���ĿΪ15mol��6.02��1023mol-1=9.03��1024����
��3��n��H2��=4g��2g/mol=2mol��n��O2��=11.2L��22.4L/mol=0.5mol��n��H2O��=![]() =
=![]() mol���������ʵ���Խ������������Խ�࣬����������������4gH2��4gH2������ԭ�����ʵ���Ϊ2mol��2=4mol����״����11.2LO2������ԭ�����ʵ���Ϊ0.5mol��2=1mol��1mLH2O������ԭ�����ʵ���Ϊ
mol���������ʵ���Խ������������Խ�࣬����������������4gH2��4gH2������ԭ�����ʵ���Ϊ2mol��2=4mol����״����11.2LO2������ԭ�����ʵ���Ϊ0.5mol��2=1mol��1mLH2O������ԭ�����ʵ���Ϊ![]() mol ��3=
mol ��3=![]() mol������ԭ����������4gH2����״����11.2LO2������Ϊ0.5mol��32g/mol=16g��1mLH2O������Ϊ1mL��1g/mL=1g��������С����1mLH2O����״����H2��O2����̬��H2O��Һ̬�������С����1mLH2O��
mol������ԭ����������4gH2����״����11.2LO2������Ϊ0.5mol��32g/mol=16g��1mLH2O������Ϊ1mL��1g/mL=1g��������С����1mLH2O����״����H2��O2����̬��H2O��Һ̬�������С����1mLH2O��
��4����ͬ״���£���ͬ������ij����������H2�������Ϊ1:8������ͬ�����ĸ�����������H2���ʵ���֮��Ϊ1:8������n=![]() ��������������H2��Ħ������֮��Ϊ8:1��H2��Ħ������Ϊ2g/mol���������Ħ������Ϊ16g/mol��
��������������H2��Ħ������֮��Ϊ8:1��H2��Ħ������Ϊ2g/mol���������Ħ������Ϊ16g/mol��
��5��O2��O3��Ħ����������Ϊ32g/mol��48g/mol������n=![]() ����������O2��O3���ʵ���֮��Ϊ48:32=3:2������������֮��Ϊ3:2������Oԭ�Ӹ�����Ϊ1:1����ͬ״�����������֮�ȵ�������������ʵ���֮�ȣ���ͬ״���µ����֮��Ϊ3:2��
����������O2��O3���ʵ���֮��Ϊ48:32=3:2������������֮��Ϊ3:2������Oԭ�Ӹ�����Ϊ1:1����ͬ״�����������֮�ȵ�������������ʵ���֮�ȣ���ͬ״���µ����֮��Ϊ3:2��


 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʷ���״̬�仯���˷������Ӽ�����������ͬ�����͵���
A.HCl����������ˮB.ˮ���ɱ��ۻ�
C.���ʵ�������H2S�ķֽ�D.NaF�������Ʒֱ������ۻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й���Һ����˵���������(����)
A. ��ȡ12.5g����(CuSO4��5H2O)����ˮ�У�����ˮϡ����500mL��������Һ���ʵ���Ũ��Ϊ0.1mol��L��1
B. ȡ58.5 g NaCl�������1 Lˮ�г���ܽ⣬������Һ��NaCl�����ʵ���Ũ��Ϊ1 mol��L��1
C. ��100g5%��ʳ��ˮ����������50gˮ��������Һ��NaCl����������Ϊ10%
D. ��Ũ��Ϊ2 mol��L��1��������Һ10 mL��ˮϡ����200 mL��������ҺŨ��Ϊ0.1mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ������ͨ��������CO2����,��ַ�Ӧ���������,�ٸ��³������,��ȴ�����õĹ�������Ϊ()
A. Na2SiO3 B. Na2SiO3��Na2CO3
C. SiO2��Na2CO3 D. SiO2��Na2SiO3��Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�������;��˵���У�����ȷ���ǣ� ��
A.NH3�����������
B.�ռ����θ������һ��ҩ��
C.Na2SiO3��������ľ�ķ����
D.NaHCO3��������۷��ݼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��������Ļ���������(��Ҫ�ɷ�ΪFe2O3��������FeS��SiO2��Cu��Au��Ag��)�ǹ�ҵ����֮һ�����ۺ�������һ�����Ϊ������Ҫ;����
I.�Ի���������Ϊԭ���Ʊ���������(Fe2O3)�ͻ���(NH4)2SO4������������������ͼ:
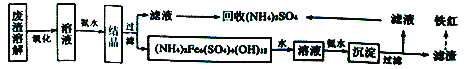
��ش���������:
(1)����ߡ������ܽ⡱���ʵĴ�ʩ��______(д��һ������)������������Ŀ����______________�� (2)(NH4)2Fe6(SO4)4(OH)12��Fe�Ļ��ϼ���__________________��
(3)�ù�������ͼ�У��ڶ��μ��백ˮ��Ӧ�����ӷ���ʽΪ________________________��
II.��ͼ���Ի���������Ϊԭ���Ʊ��������������һ��������������:
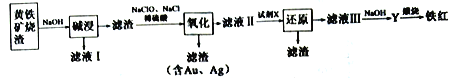
(4)����ҺI�м������۵���ʯ�ң���Ӧ�Ļ�ѧ����ʽ��___________________��
(5)����������Ӧ�϶࣬����FeS ���Կ�����ˮ��Һ��Cl2���������������Һ�еμ�BaCl2�в��ܽ�������İ�ɫ�������ɣ���ˮ��Һ��FeS ��Cl2 ��Ӧ�����ӷ���ʽΪ________________��
(6)�Լ�X Ϊ�������ۣ���������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���25mL0.1mol/LNaOH ��Һ����μ���0.2mol/LCH3COOH ��Һ����ͼ������ʾ���й�����Ũ�ȹ�ϵ�������
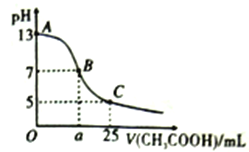
A. ��һ��һ������c(Na+)+c(H+)=c(CH3COO-)+c(OH-)
B. B����a=12.5��c(Na+ ) =c(CH3COO-)>c(OH-)= c(H+)
C. C�㣺c(CH3COO-)+ c(CH3COOH)=2 c(Na+ )=0.1mol/L
D. C����c(CH3COO-)��c(CH3COOH))=3��2����CH3COOH��Ka=1.5��10-5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������л�����ijЩҩ���е���Ч�ɷ�

����˵����ȷ���ǣ� ��
A. �����л��ﶼ�ܷ���ˮ�ⷴӦ
B. �����л��ﱽ���ϵ���ԭ��������ԭ��ȡ������һ�ȴ��ﶼֻ��2��
C. �������ʵ������������ʼ�������������Һ�У����ǻ���Ƥ�����������������
D. ʹ��FeCl3��Һ��ϡ������Լ����������л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧʵ������Ҫ0.2 mol��L-1��NaOH��Һ450 mL��0.5 mol��L-1��������Һ500 mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����__������ţ�������������Һ�����õ��IJ���������__________�����������ƣ���

��2������ƿ��������Һ����Ҫ����������ƿ�ϱ������������е� ____����д��ţ���
���¶� ��Ũ�� ������ ��ѹǿ ����ʽ���ʽ �̶���
��3������ʱ������ȷ�IJ���˳����____������ĸ��ʾ��ÿ����ĸֻ����һ�Σ���
A����30 mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ����õ��������ƹ������ձ��У��ټ�������ˮ��Լ50 mL�����ò���������������ʹ�����ܽ⣬��ȴ������
C��������ƿ�ǽ���ҡ��
D�����ܽ������������Һ�ز�����ע������ƿ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2 cm��
��4�����ݼ����֪����ʵ�����ȡNaOH���������Ϊ_______________g��
��5�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ����������___________________��
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ��С��0.5 mol��L-1���������������Щ��������������Ũ��ƫС_________________������ĸ����
A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B������ƿδ���T����������Һ
C��������ƿת��ʱ��������Һ�彦��
D��������ƿ�ж���ʱ��������ƿ�̶���
E���ձ�δ����ϴ��
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com