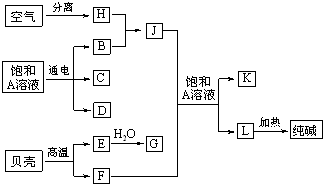
�⣺���ǵ���Ҫ�ɷ�ΪCaCO
3�����ȿ�����CaO��CO
2���ɴ����֪LΪNaHCO
3����F��J�ķ�ӦӦΪ�����Ƽ�ķ�Ӧ���ɴ˿�֪EΪCaO��FΪCO
2��GΪCa��OH��
2��JΪNH
3��AΪNaCl��KΪNH
4Cl��HΪN
2��BΪH
2��C��D��C��G��Ӧ��������ȡ����������CΪCl
2��DΪNaOH��
��1�������Ϸ�����֪CΪCl
2��DΪNaOH�����߷�Ӧ����NaCl��NaClO������NaClOΪ����������Ч�ɷ֣��ʴ�Ϊ��NaClO��
��2��AΪNaCl����ҵ�������ӷ���ʽΪ2H
2O+2Cl
-
Cl
2��+H
2��+2OH
-��
�ʴ�Ϊ��2H
2O+2Cl
-
Cl
2��+H
2��+2OH
-��
��3����F��Jͨ��A�ı�����Һ�У�Ϊ�����Ƽ�ķ�Ӧ����Ӧ�ķ���ʽΪNaCl+CO
2+NH
3+H
2O=NaHCO
3��+NH
4Cl��
�ʴ�Ϊ��NaCl+CO
2+NH
3+H
2O=NaHCO
3��+NH
4Cl��
��4��JΪNH
3��Ϊ�����νṹ���ʴ�Ϊ��������
��5��HΪN
2�����������H��ʣ�����������Ҫ�ɷ���O
2��BΪH
2��DΪNaOH����ɼ���ȼ�ϵ�أ���������������Ӧ���缫��ӦʽΪ2H
2+4OH
--4e
-�T4H
2O������������ԭ��Ӧ���缫��ӦʽΪO
2+2H
2O+4e
-�T4OH
-��
�ʴ�Ϊ��O
2��2H
2+4OH
--4e
-�T4H
2O��O
2+2H
2O+4e
-�T4OH
-��
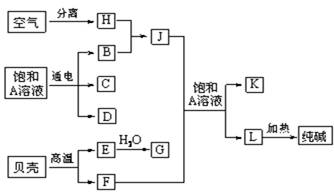
���������ǵ���Ҫ�ɷ�ΪCaCO
3�����ȿ�����CaO��CO
2���ɴ����֪LΪNaHCO
3����F��J�ķ�ӦӦΪ�����Ƽ�ķ�Ӧ���ɴ˿�֪EΪCaO��FΪCO
2��GΪCa��OH��
2��JΪNH
3��AΪNaCl��KΪNH
4Cl��HΪN
2��BΪH
2��C��D��C��G��Ӧ��������ȡ����������CΪCl
2��DΪNaOH��������ʵ����ʺ���Ŀ��Ҫ������⣮
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע����ݱ��Ǻͺ����ƼΪ�����ͻ�ƿڽ����ƶϣ�ѧϰ��ע�����ԭ��صĹ���ԭ���͵缫��Ӧʽ����д��

 �Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮
�Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮ Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-�� Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-��

 ��У����ϵ�д�
��У����ϵ�д�
 �Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮
�Ժ�ˮ�г�������AΪԭ�Ͽ��Է�չ�ܶҵ��ͼ�а�������ѧ�α��н��ܵļ��ֻ�����ҵ����������ݸ�����֮���ת����ϵ�ش��������⣮