
ͨ��״���£�X��Y��Z��������̬���ʡ�X�����Ԫ���ǵ�������ԭ�Ӱ뾶��С��Ԫ�أ�ϡ������Ԫ�س��⣩��Y��Z����Ԫ��R��ɣ���ӦY+2I-+2H+ I2+Z+H2O����ΪY�ļ�����Ӧ��
I2+Z+H2O����ΪY�ļ�����Ӧ��
(1)Y��Z�Ĺ�ϵ�ǣ�ѡ����ĸ��_______��
a.ͬλ�� b.ͬϵ�� c.ͬ�������� d.ͬ���칹��
(2)��Y�Ͷ�������ֱ�ͨ��Ʒ����Һ������ʹƷ����ɫ����������ɫ����Һ������ߵ�ʵ�鷽��_________________________________________________________________
___________________________________________________________________��
(3)�ٳ�ʵ��˵��X�������Ա����ʵ�������ǿ���û�ѧ����ʽ��ʾ����
___________________________________________________________________��
(4)���壨CN��2��X��ѧ�������ƣ�Ҳ����H2��Ӧ����HCN����ˮ��Һ��һ���ᣩ��
��HCN�����к���4�����ۼ�����ṹʽ��__________________________________��
��KCN��Һ�Լ��ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________��
(5)���������£�������Z��ij����M����MCR3��CΪ̼Ԫ�أ���ȫ��Ӧ����CR2��MmRn(m��n��Ϊ������)����CR2����Ϊ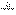 1g��MmRn����Ϊ
1g��MmRn����Ϊ 2g��M�����ԭ������Ϊa����MmRn��m:n��_____________(�ú�
2g��M�����ԭ������Ϊa����MmRn��m:n��_____________(�ú�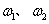 ��a�Ĵ���ʽ��ʾ)��
��a�Ĵ���ʽ��ʾ)��
���𰸡�
(1)c
(2)������ɫ�����Һ������Һ�ָ���ɫ����ԭͨ������ΪSO2������Һ����죬��ԭͨ��������O3
(3)2Fe+3Cl2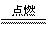 2FeCl3 Fe+S
2FeCl3 Fe+S FeS�����������𰸾��ɣ�
FeS�����������𰸾��ɣ�
(4)��H-C��N ��CN-+H2O HCN+OH-
HCN+OH-
(5)16w1��(44w2-aw1)
��������X�����Ԫ��Ϊ��������ԭ�Ӱ뾶��С��Ԫ�أ�XӦΪCl2��Y��Z����RԪ����ɣ���Y��ZΪ��RԪ����ɵIJ�ͬ�ĵ��ʣ����ǻ�Ϊͬ�������塣��Y+2I-+2H+ I2+Z+H2O������Y�ļ�����Ӧ��֪YΪO3��ZΪO2��
I2+Z+H2O������Y�ļ�����Ӧ��֪YΪO3��ZΪO2��
��1��Y��Z�Ĺ�ϵΪͬ�������壬��C��
��2��O3��SO2����ʹƷ����Һ��ɫ����O3����Ʒ�췢��������ԭ����ɫ��SO2����Ʒ�췢���������ɲ��ȶ�����ɫ���ʶ���ɫ�����ߵ���������SO2ʹ����ɫ����Һ���ȶ������Ⱥ���Իָ�ԭ����ɫ����O3ʹ����ɫ����Һ�����ٻָ�����˿��Լ��ȶ�����ɫ�����Һ�����ָ�ԭ����ɫ��ΪSO2����֮��ΪO3��
��3������������ԭ��Ӧ��ԭ������ѧ���ij�����ѧ��Ӧ���÷�Ӧ����Ϊ��H2S+Cl2 S+2HCl��2Fe+3Cl2
S+2HCl��2Fe+3Cl2 2FeCl3��Fe+S
2FeCl3��Fe+S FeS�ȡ�
FeS�ȡ�
��4����HCN�����к���4�����ۼ�������H��C��NԪ�ص�ԭ�ӽṹ�ص��֪����ӽṹΪH��C��N��
��KCN�����Σ���ˮ��Һ�Լ��ԣ�˵��HCN�����ᣬ����Һ��CN-����ˮ�⣺CN-+H2O HCN+OH-��
HCN+OH-��
��5�������֪��
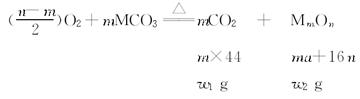
����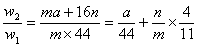


��m��n=16w1��(44w2-aw1)


 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪�������ԣ�
 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ� ��I����
��I����
3I2��6OH��
 ��5I����3H2O��
��5I����3H2O��
KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������_________���û�ѧʽ��ʾ����CCl4�����Ϻ�ɫ��������___________________���õ���ʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ___________________________��______________________________________��
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��
д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_____________________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ�______����ǡ�������˵������________________________________________��
��3��Ϊ����ӵ��Σ�����KI�����ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ�������___________________��
A��Na2S2O3 B��AlCl3 C ��Na2CO3 D��NaNO2
��4���Ժ�Fe2���϶��ʳ��(���費��Fe3��)����ѡ��KI��Ϊ�ӵ���������ʵ�鷽��������üӵ����е�Fe2����__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
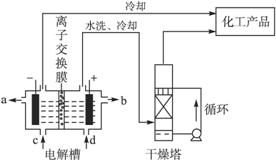
��ҵ�ϵ�ⱥ��ʳ��ˮ����ȡ���ֻ���ԭ�ϣ����в���ԭ�Ͽ������Ʊ��ྦྷ�衣
��1����ͼ�����ӽ���Ĥ����ⱥ��ʳ��ˮʾ��ͼ����������������������____________��NaOH��Һ�ij���Ϊ___________������ĸ�������Ʊ���ʳ��ˮ�Ľ���Ϊ___________������ ĸ������������Ӧʹ�õ�Һ����___________��
ĸ������������Ӧʹ�õ�Һ����___________��
��2���ྦྷ����Ҫ����SiHCl3��ԭ�����������丱����SiCl4���ۺ������ܵ��㷺��ע��
��SiCl4���������̿�ڣ�����ά��Ҫԭ����ͬ��������Ϊ������SiCl4��H2��O2��Ӧ�����������֣���ѧ����ʽΪ____________________________________________��
��SiCl4��ת��ΪSiHCl3��ѭ��ʹ�á�һ�������£���20 L�����ܱ������еķ�Ӧ��
3SiCl4��g��+2H2��g��+Si��s�� 4SiHCl3��g��
4SiHCl3��g��
��ƽ���H2��SiHCl3���ʵ���Ũ�ȷֱ�Ϊ0.140 mol/L��0.020 mol/L����H2ȫ����Դ�����ӽ���Ĥ���ĵ���������������Ĵ�NaCl������Ϊ___________kg��
��3��������Ĥ���۵�ⱥ��ʳ��ˮ������ȡ�����ƣ�ͬʱ�������������Ƶ�������213.0 kg������������_________m3����״������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����ñ�ɫ���ⶨ������������淋Ĵ��ȵ�˵����ȷ����(����)
A�����Ʊ�ɫ��ʹ�õĵζ����Ǽ�ʽ�ζ���
B���ڲ�ͬ����ı�ɫ����Fe3��Ũ�Ȳ��Խ�ⶨ����������淋Ĵ������ԽС
C�����Ʊ�ɫ��ʱ������һ�����������Ŀ��������Fe3����ˮ��
D���ô����Ʒ��Һ���ɫ�ױȽϣ�����ȷȷ����Ʒ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о����֣�������������NO2�ܲ���������������γɣ���Ӧ�������£�
 ��SO2+NO2
��SO2+NO2 SO3+NO
SO3+NO
��SO3+H2O H2SO4
H2SO4
��2NO+O2 2NO2
2NO2
NO2�����������е����ã���H2SO4�������仯�е��������Ƶ��ǣ� ��
A.��ʪ������ͨ��ʢ��ŨH2SO4��ϴ��ƿ B.����ͨ��ŨH2SO4��
C.ŨH2SO4����өʯ�У����� D.��������H2SO4ʹ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ���������( )��
�ٶ�����̼����Ҫ�Ļ���ԭ��
�ڶ�����̼��ֲ�������õı���ԭ��
�۶�����̼����ɫ����ζ����������
�ܳ�������̼�⣬���顢һ��������Ҳ����������
A.�٢� B.�ڢ�
C.�ۢ� D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�û�ѧ���ű�ʾ����4������
A�� B �� C�� D ��
(2)д��A��B��E��������Ӧ�����ӷ���ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ�����г����в���(H2O)2��Ҫȷ��(H2O)2�Ĵ��ڣ��ɲ��õķ�����
A��1Lˮ���������������������Ʒ�Ӧ����������������
B��1Lˮ����ͨ��Ũ�����Ũ�������ص�����
C����ˮ����������ˮ��pH
D����ˮ��������������ԭ�ӱ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com