
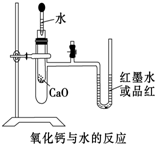
 ��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ�
��ͼ��ijͬѧ��Ƶķ��ȷ�Ӧ�Ĺ۲�װ�ã���ʵ��˳���ǣ�

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
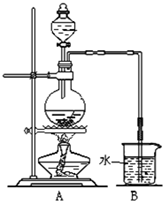 ��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
��ͼ��ij�о���ѧϰС������ʵ������������װ�������ȡ��ˮ���������ʵ�飮
| ||
| ||
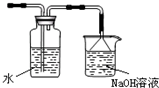 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a | b | c | d | |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | ��ʯ�� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����һ��2009��ڶ�����ʱѵ�����ۻ�ѧ(4) ���ͣ�058
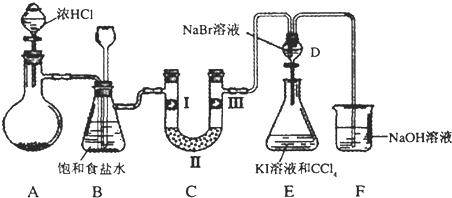
��ͼ��ij��ѧ��ȤС���ͬѧ��Ƶ���ȡNH3������NH3����ʵ��ĸĽ�װ�ã������ȳ�ȡmg�����Ȼ�立����Թܵײ����ٿ��ٳ�ȡng�������ƹ�������Ȼ�隣��棬�����ô��ιܵĽ��������Թ�(�ι�����������һ�������Ũ��ˮ)��������ѹ��ͷ�ιܣ����������Թ��ڲ����������ݣ���ش��й����⣺
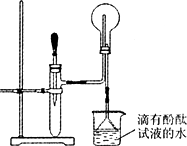
(1)�û�ѧ��ȤС���ͬѧ���ô�װ����ȡNH3��ԭ�������˷���������Ϊ��������(�����)��________��
��Ũ��ˮ�д���ƽ�⣺NH3��H2O![]() NH3��H2O
NH3��H2O![]() NH+4��OH����NaOHʹƽ�������ƶ���
NH+4��OH����NaOHʹƽ�������ƶ���
��Ũ��ˮ�д���ƽ�⣺NH3��H2O![]() NH3��H2O
NH3��H2O![]() NH+4��OH����NH4Clʹƽ�������ƶ���
NH+4��OH����NH4Clʹƽ�������ƶ���
��NaOH����ˮʱ���ȣ��¶����ߣ�NH3���ܽ�ȼ�С���в���NH3�ݳ���
��NH4Cl��NaOH�ڴ������·�Ӧ������NH3��
(2)�����ж�ͼ����ƿ������NH3������________��
(3)��ʵ�黹��֤��NH3��ʲô���ʣ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com