
��12�֣��±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
|
�� ���� |
IA |
|
0 |
|||||
|
1 |
�� |
��A |
��A |
��A |
��A |
��A |
��A |
|
|
2 |
|
|
|
|
|
�� |
�� |
|
|
3 |
�� |
|
�� |
|
|
�� |
�� |
|
��1���ܡ��ݡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________��
��2���͢ߵ���ۺ����������ǿ��Ϊ__ ___>_______��
��3���١�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������ĵ���ʽΪ__________����������Һ�и������ܽ�Fe2+������д���÷�Ӧ�����ӷ���ʽ____________��
��4���ɱ���Ԫ���γɵ����ʿɷ�����ͼ�еķ�Ӧ������B��C��G�ǵ��ʣ�BΪ����ɫ���壬D��Һ�Լ��ԡ�

�� д��D��Һ��G��Ӧ�Ļ�ѧ����ʽ____________��
�� д������A��Һ�����ʵ������ӵķ���: ____________��
�� �����£������1 L 0.1 mol/L A��Һ��һ��ʱ�� ������ҺpHΪ12��������Һ����仯������õ�������ת�Ƶ��ӵ����ʵ���Ϊ__________ mol��
�� ����ͼ�и�����Ӧ��Ϊ��ȫת����������X�к��е�������_______��
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| a | |||||||||||||||||
| f | y | h | i | ||||||||||||||
| b | e | j | |||||||||||||||
| c | d | g | l | ||||||||||||||




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� ���� |
IA | 0 | ||||||
| 1 | �� | IIA | IIIA | IVA | VA | VIA | VIIA | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
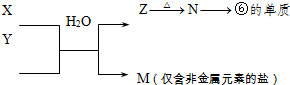
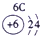
| ��һ���� | 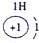 |
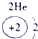 | ||||||
| �ڶ����� |  |
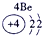 |
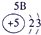 |
 |
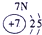 |
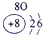 |
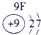 |
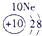 |
| �������� | 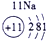 |
 |
 |
 |
 |
 | ||
 ��ʾ����
��ʾ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com