
�������������������бز����ٵ���Ҫ������
��1����Ȼ��һ�����������ȶ�����ԭ�ӣ��ֱ���54Fe��56Fe��57Fe��58Fe������58Feԭ����������������֮��Ϊ___________��
������Ԫ�صĽ�����������ļ���ʽΪ��54��a1%+56��a2%+57��a3%+58��a4%������a1%��a2%������ָ��ͬλ�ص�____________________��
��2����ԭ������______��������ͬ�ĵ��ӣ���ԭ�Ӵ����ĵ�������_____�ֲ�ͬ����չ����
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�������������ǿ�Ļ�����Ļ�ѧ��Ӧ����ʽΪ________________________________��
��4��Ҫ����Ƭ��пƬ��ֱ����Դ�͵��������Ƭ��п��ʵ�飬��ƬӦ����_________�����������Һ��____________________��
��5���������ƣ�Na2FeO4����ˮ����������ʹ�õ�һ�����;�ˮ�������������Աȸ�����ظ�ǿ�������ڷ�Ӧ�б���ԭΪFe3+����ƽ��ȡ�������ƵĻ�ѧ����ʽ��
___Fe(NO3)3 + ___NaOH + ___Cl2 ��___Na2FeO4 + ___NaNO3 + ___NaCl + ___H2O
�������Ƴ���������ɱ���⣬��������ˮ�е��������ԭ����______________________��
��6��0.03mol�����ӵ�������HNO3�У����ȣ�����ȫ�ܽ⣬������NO��NO2�Ļ�����干1.12L����״��������ʢ�д����������������ˮ�У�ͨ���״����һ�������O2��ǡ��ʹ����ȫ������ˮ����HNO3����ͨ��O2�����________________L��
��1��6 ��ȣ���1�֣�
��2��7 9����1�֣�
��3��KOH + HBrO4 ��H2O + HBrO4 ��2�֣�
��4���� ZnCl2��Zn ������Һ�����ԣ� ����1�֣�
��5��2 16 3 2 6 6 8 �� Fe3+ˮ�⣬������Һ�е����ʣ���1�֣�
��6��0.504L�� 2�֣�
���������������1��58Feԭ���У�������Ϊ26��������Ϊ32������֮��Ϊ6��a1%��a2%������ָ��ͬλ�ص�����Ȼ���е�ԭ�Ӱٷֺ���������ȡ�
��2��������ԭ�ӵĺ�������Ų�ʽ��֪����7��������ͬ�ĵ��ӣ���ԭ�Ӵ����ĵ��Ӽ���3���Ӳ㣬��������9�ֲ�ͬ����չ����
��3������ͬ���ڵ�����Ԫ���У�����������Ӧˮ�����У�������ǿ�Ļ�����������������������ǿ�Ļ����� HBrO4����Ӧ�Ļ�ѧ��Ӧ����ʽΪKOH + HBrO4 ��H2O + HBrO4��
��4��Ҫ����Ƭ��пƬ��ֱ����Դ�͵��������Ƭ��п��ʵ�飬��ƬӦ������ �����������Һ��ZnCl2��Zn ������Һ�����ԣ���
��5�����ݻ��ϼ�����������ȣ�������ƽ���������Ƴ���������ɱ���⣬��������ˮ�е��������ԭ���� Fe3+ˮ�⣬������Һ�е����ʡ�
��6��NO2��NO�Ļ������Ϊ0.05mol���� O2��Ϻ�ͨ��ˮ�У���������ǡ����ȫ��ˮ�����������ᣬ�����ṩ�ĵ������ʵ�������������õ�����ӵ������ʵ�������ʧȥ���ӱ�����������ӣ�ʧ����Ϊ0.09mol�����������ʵ���Ϊ0.09/4mol,���Ϊ0.504L��
���㣺���⿼�������ӵ��Ų���������ԭ����ƽ�������غ㷨���û�ѧ��������֪ʶ��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ȡ��������Ŀ�������Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��Al2O3��SiO2�ȣ��Ʊ��ߴ�����������-Fe2O3���Ĺ����������£�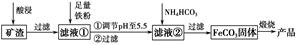
��1���������������Fe3O4������Ӧ�����ӷ���ʽΪ______________________________��Ϊ��ߡ����������Ԫ�صĽ����ʣ����˲��ú��ʵ�Һ�̱Ⱥ�ѭ����ȡ�⣬���˵���������____________________��____________________�����ξ�������
��2������pH��5.5��Ŀ����______________________________________��
��3����Һ���м���NH4HCO3ʱ��Ҫ���Ʒ�Ӧ�¶Ȳ��ܹ��ߣ�ԭ����__________________________________________________������һ�㼴�ɣ�
��4���ڿ���������FeCO3�Ʊ��ߴ��������Ļ�ѧ����ʽΪ_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ķ����ϣ��ش��������⡣
���ϣ����������Ƽ���Ա�о��õ�һ�����Ͳ��ϡ�����ĭ�������ǰѷ��ݼ��ӵ��������Ͻ����Ƴɵģ����ŵ���Ӳ�ȸߣ��ܶ�С(ԼΪ0.16��0.5 g/cm3)����ľ�Ļ��ᣬ�ɸ���ˮ�棬���кܴ���ԣ��Ҹ��������£���һ�����õĽ������Ϻ����ʲ��ϣ�������ɴ�����Ͷ���г���
��1�����й�����ĭ����˵��������� ��
A����ĭ��������������ĭ
B����ĭ����һ�ֺϽ�
C����ĭ����һ�����ʵĽ������Ϻ����ʲ���
D����ĭ�������ڷɻ�����
��2�����Ƴ�������ʳƷ��װ��������������һ���� ��
A���������� B����չ�� C�������� D��������
��3�����ڿ����лᱻ��������һ�����ܵ�����Ĥ(������)�������𱣻����ã����������Ĥ����ǿ���ǿ����ܽ⣬��д����
�������ᷴӦ�����ӷ���ʽ�� ��
��������������Һ��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��2.0 mol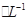 CuSO4��1.0 mol
CuSO4��1.0 mol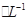 H2SO4��Һ�������ϣ������Ϻ����Һ��������ڻ��ǰ������Һ�����֮�ͣ����㣺
H2SO4��Һ�������ϣ������Ϻ����Һ��������ڻ��ǰ������Һ�����֮�ͣ����㣺
��1�������Һ��CuSO4��H2SO4�����ʵ���Ũ��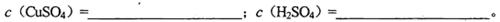
��2�����Һ�к͵����ʵ���Ũ��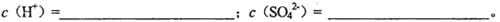
��3������Һ�м������ۣ������㹻����ʱ�䣬������ʣ�ࡣ��ʱ��Һ�е����ʵ���Ũ�ȡ�c(Fe2��)=_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ͭ���仯�������������������й㷺����;���Իش��������⣺
��1������ԭ�ӽṹʾ��ͼΪ___________����������ͭ���ֽ����Ľ������������ǿ������˳����__________��
��2�������·��Ĺ����У�FeCl3��Һ����ʴ��ͭ������д���÷�Ӧ�����ӷ���ʽ��_________________��
��3����ͼװ���У�����________����ͭ�缫�ĵ缫��ӦʽΪ_________________________��
��4����֪ͭ��ϡ�����Ӧ����ͭƬ��ϡ�����г�ʱ�����ʱ��Һ�����ɫ�����û�ѧ����ʽ��ʾ��ԭ��__________________________________________________________��
��5���Ȼ����㷺�����л��ϳɺ�ʯ��ҵ�Ĵ��������������(��Ҫ�ɷ�ΪA12O3)�뽹̿��Ϻ���Ȳ�ͨ���������ɵõ��Ȼ�����ͬʱ����CO��д���÷�Ӧ�Ļ�ѧ����ʽ��_____________________________ ���÷�Ӧ����������_________________��
��6��ijУ��ȤС��Ϊ�ⶨһ��������Ͻ� (FexAlySiz) ��ĩ����ɣ�������·�����ȷ��ȡ1.46 g�úϽ��ĩ���������������Һ����ַ�Ӧ����ˣ��ⶨʣ���������0.07 g������Һ�еμ�����NaOHŨ��Һ����ֽ��衢���ˡ�ϴ�ӵù��塣�ٽ����ù����ּ��ȡ����գ��ú���ɫ��ĩ1.60g��ͨ������ȷ���˺Ͻ�����Ϊ________________ (�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����һƿ���ʱ��ϳ�����������������ϲ������Ա仯����ijѧ���������в��������������ʣ��������1���������������Ƿ���ʵ�ʵ�鷽���� ������б��ʣ�����������������Һʱ��Ӧ��γ�ȥ���ʵ����ʣ� ��
��2��FeCl3��Һ�ػ�ɫ���Դ���Һ���ֱ�������ʵ�飬�������
| ��� | ʵ������ | ʵ����Ҫ���� | ���ӷ���ʽ |
| �� | ����������� | | |
| �� | ��������Na2O2��ĩ | | |
| �� | ��������AgNO3��Һ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ��������Ĺ�������ʾ��ͼ���£�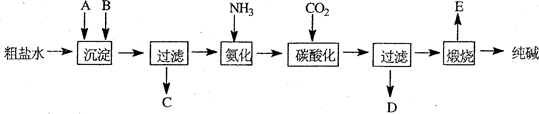
���������գ�
��1������ˮ���������A��B������(������A��Դ��ʯ��Ҥ��)��д��A��B�Ļ�ѧʽ��
A ��B ��
��2��̼�ữ������Ӧ�Ļ�ѧ����ʽ�ǡ���������������������������������������
��3����ĸҺ ��ѡ��ͼ����ĸ����ͨ����������ϸСʳ�ο�������ȴ��������Ʒ��ͨ�����������С�������
A������NH4����Ũ�ȣ�ʹNH4Cl���������
B��ʹNaHCO3���������
C��ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
��4�����������Լ�����鸱��ƷNH4Cl�Ƿ��ķ����������ǡ� .
��5��Xg�����Ʒ(����̼������)��ּ��ȷֽ������������Yg������Ʒ��̼�����Ƶ����������ɱ�ʾΪ ��
��6�������������ƺͽ�̿��ʯ��ʯ�ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô����Ӧ�Ļ�ѧ����ʽΪ_________ __ ����֪����֮һΪCaS����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ������1.00mol/LHCl��Һ������HCl��Һ����������ɳ����Ĺ�ϵ��ͼ��ʾ��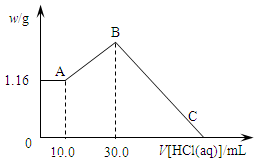
��1��A��ij�����Ļ�ѧʽΪ ��
��2��д��A����B�㷢����Ӧ�����ӷ���ʽ�� ��
��3��ԭ�������NaOH�������� g��C�㣨��ʱ����ǡ����ȫ�ܽ⣩HCl��Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ϸ��������ˮ��Һ�����������£����Խ���ͭ����Ҫ�ɷ���CuFeS2������������SiO2�������������Ρ����ø�ԭ������ͭ���̷���FeSO4��7H2O�����������£�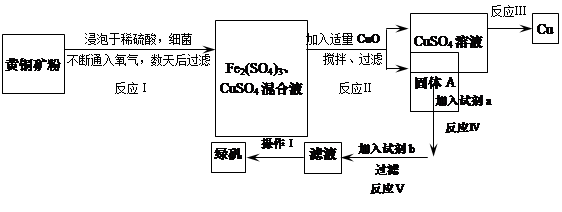
�ش��������⣺
��1����֪��
| | Fe2+ | Cu2+ | Fe3+ |
| ��ʼת���������������ʱ��pH | 7.6 | 4.7 | 2.7 |
| ��ȫת���������������ʱ��pH | 9.6 | 6.7 | 3.7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com