
ijѧ�������������֤�Ҷ��ᣨHOOC��COOH��(�׳Ʋ���)��һЩ���ʵ�ʵ�飬����ʵ��ش��������⣺
��1����pHֵ��ֽ��֤�Ҷ��������,�����������: ��
��2����֪��������175��ʱ���ȷֽ������H2O��![]() ��CO��
��CO��
��ѧ���������ͼʵ��װ�ã���֤������ֽ��������CO��֤������������ش��й����⣺
|
|
������ʵ��װ��������ϴ��ƿ�ֱ�ʢ�г���ʯ��ˮ������������Һ��Ũ���ᣬ���Ӧʢ ����Ӧʢ ��
�ڱ��в������е�����Ϊ�� ��
�۶�װ�������� ��
����ʵ������У���ѧ������Ӧ������ʵ��װ�ý���һЩ�Ľ������ܱ�֤ʵ��Ŀ�ѧ�ԺͰ�ȫ�ԣ���ѡװ������ͼ������ش��������⣺
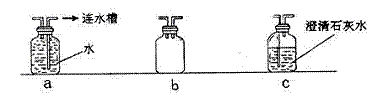
��Ϊȷ֤ͨ�������ܵ�CO�������ѳ�ȥCO2�����ڼ���֮������ ����������װ����ţ�
����װ�ñ��Ͷ�֮������bװ�ã��������� ��
�۴ӻ��������ĽǶȿ���Ӧ����β������װ�ã�����ν���β������ ��
��1����pHֵ��ֽ��֤�Ҷ��������,����������ǣ�ȡһ��pHֵ��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ��Һ����pH��ֽ���в�������Ӻ����ɫ����Ƚϣ��ó�pHֵ
��NaOH��Һ Ũ����
�ڲ������к�ɫ������
����֤CO����������CO2
��2����c
�ڷ�ֹ���е�Һ�嵹����ʹ���е���ը��
�� ��β�����ڴ�����һȼ���ŵľƾ��ƣ���β��ȼ�մ�����
��1��ȡһ��pHֵ��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ��Һ����pH��ֽ���в�������Ӻ����ɫ����Ƚϣ��ó�pHֵ
�� ��β�����ڴ�����һȼ���ŵľƾ��ƣ���β��ȼ�մ�������������������������


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ʽ�Ȼ�ͭ��Cupric Chloride��������ˮ����ɫ�ᾧ����һ��ũҩ������ʽ��CuCl2?3Cu(OH)2?xH2O(x=1/2��1��2)��Ϊ��֤����ɲ�ȷ��Xֵ��ijѧ����������¼���ʵ�飺
��ȡa g�ľ����гɷ�ĩ��
�ھ����ڿ����м�����ȷ���������ٱ仯Ϊֹ��ͭ��Ϊ���ۣ�����ȴ���������Ϊb g��
����ȡa g�ľ��壬��������ij��Һ�г���ܽ�õ���Һ��
����۵õ�����Һ�м�����������������Һ�����õ������������Ϊc g��
�����ϲ�������ȷ���Իش��������⣺
��1������ʵ�����õ��������IJ����� ____ ������ţ���
��2��������о���Ӧ���� _�����������ƣ������գ����պ�õ��Ĺ���ӦΪ______ _____(�ѧʽ)��
��3����������õ���Һ������ ��
��4���������Ҫ����������������������Һ�� �� �� ���ɡ�������
��5������ɷ���ͨʽ������Լ���x������������� ____ ������ţ���
A��a��b B��a��c C��b��c D��a��b��c ȱһ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʽ�Ȼ�ͭ��Cupric Chloride��������ˮ����ɫ�ᾧ����һ��ũҩ������ʽ��CuCl2��3Cu(OH)2��xH2O(x=1/2��1��2)��Ϊ��֤����ɲ�ȷ��Xֵ��ijѧ����������¼���ʵ�飺
��ȡa g�ľ����гɷ�ĩ��
�ھ����ڿ����м�����ȷ���������ٱ仯Ϊֹ��ͭ��Ϊ���ۣ�����ȴ���������Ϊb g��
����ȡa g�ľ��壬��������ij��Һ�г���ܽ�õ���Һ��
����۵õ�����Һ�м�����������������Һ�����õ������������Ϊc g��
�����ϲ�������ȷ���Իش��������⣺
��1������ʵ�����õ��������IJ����� ____ ������ţ���
��2��������о���Ӧ���� _�����������ƣ������գ����պ�õ��Ĺ���ӦΪ______ _____(�ѧʽ)��
��3����������õ���Һ������ ��
��4���������Ҫ����������������������Һ�� �� �����ɡ�������
��5������ɷ���ͨʽ������Լ���x������������� ____ ������ţ���
A��a��b B��a��c C��b��c D��a��b��c ȱһ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2010�������ѧ��3���¿� ���ͣ�������
��ʽ�Ȼ�ͭ��Cupric Chloride��������ˮ����ɫ�ᾧ����һ��ũҩ������ʽ��CuCl2��3Cu(OH)2��xH2O(x=1/2��1��2)��Ϊ��֤����ɲ�ȷ��Xֵ��ijѧ����������¼���ʵ�飺
��ȡa g�ľ����гɷ�ĩ��
�ھ����ڿ����м�����ȷ���������ٱ仯Ϊֹ��ͭ��Ϊ���ۣ�����ȴ���������Ϊb g��
����ȡa g�ľ��壬��������ij��Һ�г���ܽ�õ���Һ��
����۵õ�����Һ�м�����������������Һ�����õ������������Ϊc g��
�����ϲ�������ȷ���Իش��������⣺
��1������ʵ�����õ��������IJ����� ____ ������ţ���
��2��������о���Ӧ���� _�����������ƣ������գ����պ�õ��Ĺ���ӦΪ______ _____(�ѧʽ)��
��3����������õ���Һ������ ��
��4���������Ҫ����������������������Һ�� �� �� ���ɡ�������
��5������ɷ���ͨʽ������Լ���x������������� ____ ������ţ���
A��a��b B��a��c C��b��c D��a��b��c ȱһ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʽ�Ȼ�ͭ��������ˮ����ɫ���壩��һ��ũҩ������ʽ��CuCl2��3Cu(OH)2��XH2O(X=1/2��1��2)��Ϊ��֤����ɲ�ȷ��Xֵ��ijѧ����������¼���ʵ�飺�� ȡa g�ľ����гɷ�ĩ���� �����ڿ����м�����ȷ���������ٱ仯Ϊֹ��ͭ��Ϊ���ۣ�����ȴ���������Ϊb g���� ��ȡa g
�ľ��壬��������ij��Һ�г���ܽ�õ���Һ���� ��۵õ�����Һ�м�����������������Һ��
���õ������������Ϊc g��
�����ϲ�������ȷ���Իش��������⣺
��1������ʵ�����õ��������IJ����� ____ ������ţ���
��2��������о���Ӧ����______�����������ƣ������ա�
��3����������õ���Һ������ ��
��4���������Ҫ����������������������Һ�� �� �� ���ɡ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com