
������Ԫ�صĵ���X��Y��Z��ͨ��״���¾�Ϊ��̬����������ת����ϵ����Ӧ������ȥ����
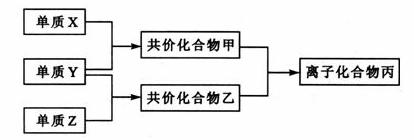
��֪��a.����˫ԭ�ӵ��ʷ����У�X���Ӻ����ۼ���ࡣ
b.���Ӻ�10�����ӣ��ҷ��Ӻ�18�����ӡ�
��1��X�ĵ���ʽ��______________________________��
��2��ʵ���ҿ�����ͼ��ʾװ�ã�ȱ���ռ�װ�ã��г̶ֹ�װ����ȥ���Ʊ����ռ��ס�

����ͼ�з������������ƿ�ռ�������װ�ü�ͼ��
���Թ��е��Լ��ǣ���д��ѧʽ��________________________________________��
���ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��
______________________________________________________________________��
��3������Z��ȼ�տ��������ֲ������һ�ֲ��ﶡ�����и�ԭ������㲻ȫ��8��
�ӽṹ�����Ļ�ѧʽ��____________________��
��4��n mol����n mol����һ�������·�Ӧ������4n mol�Һ���һ������û������������ܶ�����ͬ״����������174�����仯ѧʽ��____________________��
��1��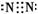
��2����
��NH4Cl��Ca��OH��2�����������ɣ�
��NH3��H2O
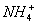 +OH-
+OH-
��3��PCl5
��4��P3N3Cl6
��������������һ������ѧ�ƶ��⡣������֪��ΪNH3����ΪHCl��XΪN2��YΪH2��ZΪCl2����ΪNH3��������ˮ���ܶȱȿ���С���������������ռ���2NH4Cl+Ca(OH)2 CaCl2+2NH3��+2H2O����ˮ�ʼ��ԣ������̪��Һ��죬NH3��H2O
CaCl2+2NH3��+2H2O����ˮ�ʼ��ԣ������̪��Һ��죬NH3��H2O
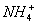 +OH��������Cl2��ȼ������PCl3��PCl5���ɶ���������㲻ȫ��8���ӽṹ֪��ΪPCl5���û������������ܶ�����ͬ״����������174��������Է�������Ϊ174��2=348������
+OH��������Cl2��ȼ������PCl3��PCl5���ɶ���������㲻ȫ��8���ӽṹ֪��ΪPCl5���û������������ܶ�����ͬ״����������174��������Է�������Ϊ174��2=348������ ��û�������N��P������ͬ����Ϊ(NP)xCly
��û�������N��P������ͬ����Ϊ(NP)xCly
45��x+35.5��y=348 x=3 y=6
��ѧʽΪP3N3Cl6
��ӦʽΪ3PCl5+3NH4Cl P3N3Cl6+12HCl
P3N3Cl6+12HCl



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(N2H4)������������һ����Ҫ�Ļ��ȼ�ϣ�N2H4��N2O4��Ӧ�ܷų��������ȡ�
(1)��֪��2NO2(g)====N2O4(g) ��H��-57.20 kJ��mol-1��һ���¶��£����ܱ������з�Ӧ2NO2(g)  N2O4(g)�ﵽƽ�⡣
N2O4(g)�ﵽƽ�⡣
������������ʱ�����д�ʩ�����NO2ת���ʵ���______________(����ĸ)��
A.��СNO2��Ũ��
B.�����¶�
C.����NO2��Ũ��
D.�����¶�
(2)25 ��ʱ��1.00 g N2H4(l)������N2O4(l)��ȫ��Ӧ����N2(g)��H2O(l)���ų�19.14 kJ����������Ӧ2N2H4(l)+N2O4(l)====3N2(g)+4H2O(l)�Ħ�H��____________________kJ��mol-1��
(3)17 �桢1.01��105 Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c(NO2)��0.030 0 mol��L-1��c(N2O4)��0.012 0 mol��L-1�����㷴Ӧ2NO2(g)  N2O4(g)��ƽ�ⳣ��K��
N2O4(g)��ƽ�ⳣ��K��
(4)����һ������Cu��������ŨHNO3��Ӧ���Ƶ�1.00 L�Ѵﵽƽ���N2O4��NO2�Ļ������(17 �桢1.01��105 Pa)������������������Cu���ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϡ�
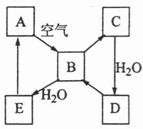
��1��д��A�ڼ�����������H2��Ӧ�Ļ�ѧ����ʽ
��2��д��E��A���⻯�ﷴӦ����A�Ļ�ѧ����ʽ
��3��д��һ����D����B�Ļ�ѧ����ʽ ;
��4����5mL0.10mol��L-1��E��Һ��10mL0.10mol��L-1��NaOH��Һ��ϡ�
��д����Ӧ�����ӷ���ʽ ;
�ڷ�Ӧ����Һ��pH 7������ڡ�����С�ڡ����ڡ����������� ;
�ۼ��ȷ�Ӧ�����Һ����pH ���� �����������䡱��С������������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йع�ҵ������������ȷ����
A.�ϳɰ����������н�NH3Һ�����룬�ɼӿ�����Ӧ���ʣ����N2��H2��ת����
B.���Ṥҵ�У��ڽӴ��Ұ�װ�Ƚ�������Ϊ������SO3ת��ΪH2SO4ʱ�ų�������
C.��ⱥ��ʳ��ˮ���ռ�������ӽ���Ĥ�����ɷ�ֹ������������Cl2����������
D.��⾫��ͭʱ��ͬһʱ���������ܽ�ͭ����������������ͭ������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�С�������͡������Ƽ�����ֹ��ա��밴Ҫ��ش����⣺
��1�����������������CaCl2�������д���ù����в���CaCl2�Ļ�ѧ����ʽ��
 ��
��
��2��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ��
��
��
��3��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ���롰�������CO2����Դ�кβ�ͬ��
 ��
��
��4����ɫ��ѧ����Ҫԭ��֮һ����߷�Ӧ��ԭ�������ʡ����ݡ������Ƽ���ܷ�Ӧ���г�����ԭ�������ʵı���ʽ��
ԭ�������ʣ�%��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�

ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL30%KOH ��Һ.������ˮԡ�У� �� ���Թ���ʢ��15mL 8 % NaOH ��Һ.�����ڱ�ˮԡ�У� �� ���Թ��������ɫʯ����Һ�� �� Ϊβ������װ�á�
����д���пհף�
��1����ȡ����ʱ������ƿ�����һ�����Ķ�������.ͨ��________________����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ� ��� ֮�䰲װʢ��___________����д���б����ĸ���ľ���װ�á�
A.��ʯ�� B.����ʳ��ˮ C.Ũ���� D.����̼��������Һ
��2���Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�_______________________________
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���________����д�����ĸ�����Ӣڵ��Թ��з�����þ���ķ�����________________(��дʵ��������ƣ�
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ�ǣ�_____________________________
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ____ɫ | ������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________________________ |
| Ȼ����Һ����ɫ��Ϊ____ɫ | _______________________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
U��V��W��X��Y��Z��ԭ������������������ֳ���Ԫ�ء�Y�ĵ�����W2��ȼ�յIJ����ʹƷ����Һ��ɫ��Z��WԪ���γɵĻ�����Z3W4���д��ԡ�U�ĵ�����W2��ȼ�տ�����UW��UW2�������塣X �ĵ�����һ�ֽ������ý�����UW2�о���ȼ�����ɺڡ������ֹ��塣
�ĵ�����һ�ֽ������ý�����UW2�о���ȼ�����ɺڡ������ֹ��塣
��ش��������⣺
��1��V�ĵ��ʷ��ӵĽṹʽΪ ��XW�ĵ���ʽΪ ��ZԪ�������ڱ��е�λ���� ��
��2��UԪ���γɵ�ͬ��������ľ������Ϳ����ǣ�����ţ� ��
��ԭ�Ӿ��� �����Ӿ��� �۷��Ӿ��� �ܽ�������
��3��U��V��W�γɵ�10�����⻯���У�U��V���⻯��е�ϵ͵��ǣ�д��ѧʽ��
��V��W���⻯����ӽ��H+������ǿ���ǣ�д��ѧʽ�� ����һ�����ӷ���ʽ����֤�� ��
��4��YW2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����VW���йط�Ӧ�����ӷ���ʽΪ ���ɴ˿�֪VW��YW2��ԭ�Խ�ǿ���ǣ�д��ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Dz���������Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵ�H2O����ȥ������֪��(a)A��B��C��D�Ƿǽ������ʣ�����B��C��D�ڳ��³�ѹ�������塣(b)��Ӧ�١����ǻ��������е���Ҫ��Ӧ��(c)������E���γ��������Ⱦ��֮һ��������K�dz��õĵ��ʡ�(d)������L����Ư���ԣ�����Cl2��NaOH��Һ��Ӧ���Ƶá�(e)������J������Ԫ����ɣ�����Է�������Ϊ32��
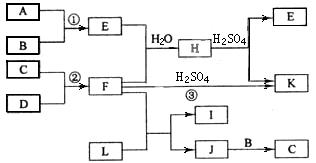
�밴Ҫ����գ�
�ŷ�Ӧ�۵Ļ�ѧ����ʽ__________________________��
��C�Ľṹʽ______________��H�Ļ�ѧʽ______________��
��L����Һ�뻯����E��Ӧ�����ӷ���ʽ__________________________��
�Ȼ�����J�Ļ�ѧʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪Co2O3��������Һ���ױ���ԭ��Co2+��Co2O3��Cl2��FeCl3��I2�����������μ��������з�Ӧ��ˮ��Һ�в����ܷ������ǣ� ��
A.3Cl2+6FeI2=2FeCl3+4FeI3 B.Cl2+FeI2=FeCl2+I2
C.Co2O3+6HCl=2CoCl2+Cl2��+3H2O D.2Fe3++2I-=2Fe2++I2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com