
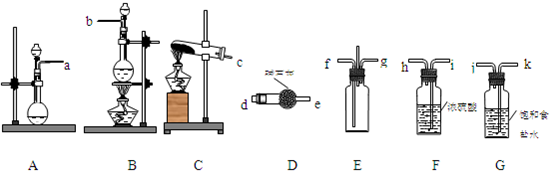
 CaCl2+2H2O+2NH3ΓϋΘΜ
CaCl2+2H2O+2NH3ΓϋΘΜ CaCl2+2H2O+2NH3ΓϋΘ°
CaCl2+2H2O+2NH3ΓϋΘ°

 ”Δ≤≈ΦΤΜ°ΤΎΡ©Βς―–œΒΝ–¥πΑΗ
”Δ≤≈ΦΤΜ°ΤΎΡ©Βς―–œΒΝ–¥πΑΗ ΨΪ”ΔΩΎΥψΩ®œΒΝ–¥πΑΗ
ΨΪ”ΔΩΎΥψΩ®œΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ

≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΫβ¥πΧβ
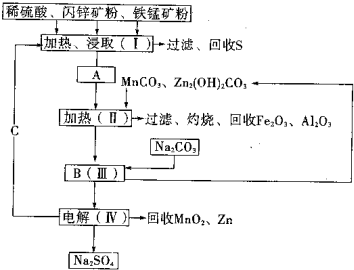 Ρ≥ΙΛ≥ß”Ο»μΟΧΩσΘ®Κ§MnO2‘Φ70%ΦΑ…ΌΝΩAl2O3Θ©ΚΆ…Ν–ΩΩσΘ®Ι≈ZnS‘Φ80%ΦΑ…ΌΝΩFeSΘ©Ι≤Ά§…ζ≤ζMnO2ΚΆZnΘ®Η…Βγ≥Ί‘≠ΝœΘ©…ζ≤ζΙΛ“’»γœ¬ΘΚ
Ρ≥ΙΛ≥ß”Ο»μΟΧΩσΘ®Κ§MnO2‘Φ70%ΦΑ…ΌΝΩAl2O3Θ©ΚΆ…Ν–ΩΩσΘ®Ι≈ZnS‘Φ80%ΦΑ…ΌΝΩFeSΘ©Ι≤Ά§…ζ≤ζMnO2ΚΆZnΘ®Η…Βγ≥Ί‘≠ΝœΘ©…ζ≤ζΙΛ“’»γœ¬ΘΚ≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com