��2011?��ɽ��ģ�⣩��֪��ͼÿһ�����е���ĸ����һ�ַ�Ӧ��������ͼ�в���������û���г�����������A����1828��¹���ѧ��ά��ͨ����������淋õ����л��Ϊ�ˣ������״������л�������ֻ���������ϸ������ȡ�ġ����������ۣ�A�Ļ�ѧʽ�ɱ�ʾΪXY
4ZM
2�����A������Ԫ�ض��Ƕ�����Ԫ�أ���ԭ������֮��Ϊ22��X��M��Z�ֱ�λ�����ڵ����壬ԭ��������������C��D��G��I��J��KΪ���壬����C��K������ЧӦ��������K�Ǻ�������ߵ��л��D��ʹʪ��ĺ�ɫʯ����ֽ������BΪһ�ְ�ɫ���壬�����ʽ�ɱ�ʾΪXY
2M
2��E�Ļ�ѧʽ�ɱ�ʾΪX
3M
4���밴Ҫ��ش��������⣮
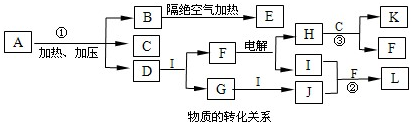
��1��AҲ��һ�ֳ��õķ��ϣ���������
���ػ�̼�����������̼����
���ػ�̼�����������̼����
��Ŀǰ��ҵ����
CO2
CO2
��
NH3
NH3
��һ���������Ƶã�
��2����Ӧ�٢ڢ۵Ļ�ѧ����ʽ�ֱ�Ϊ
6��NH2��2CO��C3H6N6+NH3��+3CO2��
6��NH2��2CO��C3H6N6+NH3��+3CO2��
��
4NO2+O2+2H2O=4HNO3
4NO2+O2+2H2O=4HNO3
��
CO2+4H2��CH4+2H2
CO2+4H2��CH4+2H2
��
��3��I��B��M����������Ϊ
66.7%
66.7%
����B��һ�����������ķ��ӣ��ӽṹ�Ͽɿ����������ӣ�XY
2M
2���ۺ϶��ɣ���һ�ֵͶ��Ļ���ԭ�ϣ�ijЩ�����������̷�������B����������������ġ�Ӥ���̷��¼������ݴˣ����㻭��B�Ľṹ��ʽ
��
II����֪�����ӵļ���Ϊ107��18�䣬���й���B��˵������ȷ����
�ܢߢ�
�ܢߢ�
��������ţ�
�ٺ��в�����̼ԭ�ӣ���һ���������ܷ����ӳɷ�Ӧ
���������ᷴӦ������
����һ���������ܷ���������Ӧ
�ܷ���������ԭ�Ӷ���ͬһƽ����
���ڸ����·ֽ�ų�N
2���ʿ�������ȼ��
����һ�������¿������������ӳɷ�Ӧ
�ߺ���������A�ĺ�����
������һ�ּ��Ի���������ڼ״�����ȩ���л���
�����ʳ�ú�B��Ⱦ�̷۵�Ӥ�����������ʯ������ϵͳ����
���ǰ�ɫ��ĩ���д̼�����ζ
��4��������E��һ�����������ϣ�����һ�ֽṹ����-X
3M
4�����п�����ʯ��������Ӳ�ȣ����Ʋ�ò��Ͽ��ܵ���;֮һ��
����ĥ���ϵ�
����ĥ���ϵ�
��
��5��Һ̬D����H
2O��Ҳ���������Ҳ�����������ͬ���������ӣ���Һ̬D���뷽��ʽΪ
2NH
3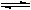
NH
4++NH
2-2NH
3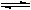
NH
4++NH
2-��
��6�����ڽṹ��N
2H
4��D�Ĺ�ϵ����H
2O
2��H
2O�Ĺ�ϵ��N
2H
4�ܷ������з�Ӧ��
N
2H
4+H
3O
+�TN
2H
+5+H
2O N
2H
4+H
2O�TN
2H
5++OH
-N
2H
5++H
2O�TN
2H
62++OH
- N
2H
5++H
2O�TN
2H
4+H
3O
+�ݴ˿ɵó��Ľ�����
C
C
��
A����ˮ�������ԡ����������������� B������ˮ�е����H
+����
C�����Ƕ�Ԫ������������������� D�����Ƕ�Ԫ����
�ڷ��䡰�����ߺš��ɴ��ij���2�Ż�������£�N
2H
4����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪4g N
2H
4��g����������Ӧ�зų�71kJ��������д���Ȼ�ѧ����ʽ
N2H4��g��+NO2��g��=3/2N2��g��+2H2O��g������H=-568kJ/mol��
N2H4��g��+NO2��g��=3/2N2��g��+2H2O��g������H=-568kJ/mol��
��


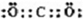
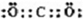 ��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬
��DΪNH3��Ϊ�����Σ�GΪNO2��Ϊ����ɫ���壬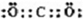 �������Σ�����ɫ��
�������Σ�����ɫ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�



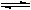 NH4++NH2-
NH4++NH2-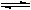 NH4++NH2-
NH4++NH2-


