
�й���Һ���������ӵļ��飬�����ж���ȷ����( )
A�����������ữ���ٵμ�KSCN��Һ���к�ɫ�������ɣ���ԭ��Һ��һ����Fe3+����
B��������������ʹ����ʯ��ˮ����ǵ��������ɣ���ԭ��Һ��һ����CO32-����
C����ij��Һ����ɫ��Ӧʱ����Ϊ��ɫ�������Һ��һ������Ԫ�أ������м�Ԫ��
D���ֱ�Mg2+��Cu2+��Fe2+��Al3+����ƿ����Һ��ֻ��NaOH��Һ����һ�μ���
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ�γ�ģ������ݣ�����ѡ������һ���������������ȫ������A�����֡�
A����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��B���⻯��ķ��ӿռ乹���� ��������ԭ�Ӳ�ȡ �ӻ���
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��E�ĺ�������Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
B��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1-�嶡��ķ�Ӧ���£�
NaBr+H2SO4 HBr+NaHSO4 ��
HBr+NaHSO4 ��
R-OH+HBr R-Br+H2O ��
R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br����Ũ��������ΪBr2�ȡ��й������б����£�
| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش��������⣺
��1�����������1-�嶡����Ʊ�ʵ���У���������������õ����� ��������ĸ��
a��Բ����ƿ b����Ͳ c����ƿ d������©��
��2���������ˮ���� ������ڡ��������ڡ���С�ڡ�����Ӧ�Ĵ�����ԭ����
��
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã������� ����ϲ㡱�����²㡱���ֲ㡱����
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��5������ȥ������е���������Br2���������������ʺϵ��� ��������ĸ��
a��NaI b��NaOH c��NaHSO3 d��KCl
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ������������� �������Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ�����������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڵ����ܺ͵縺�Ե�˵������ȷ����
A��(2012·�㽭)��һ�����ܵĴ�С��Mg��Al
B��(2012·����)��ĵ�һ�����ܸ���̼���縺�Ե���̼
C��(2012·ɽ��)Ni��Ԫ�����ڱ��е�28��Ԫ�أ��ڶ����ڻ�̬ԭ��δ�ɶԵ�������Ni��ͬ�ҵ縺����С��Ԫ����̼
D��(2013·�¿α��)F��K��Fe��Ni����Ԫ���е縺��������F
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��Ϊ������Ԫ�أ�ԭ��������������X�ĵ���Ϊ�ܶ���С�����壬Yԭ������������������������������Z��Xԭ��������������ͬ���ش��������⣺
(1)X��Y��Z��Ԫ�ط��ŷֱ�Ϊ________��________��________��
(2)������Ԫ����ɵĻ������У��Ⱥ��й��ۼ��ֺ������Ӽ�����________��________��
(3)X��Y��ɵĻ������У��Ⱥ��м��Թ��ۼ��ֺ��зǼ��Թ��ۼ�����________���˻������������������������ط�Ӧ�����ӷ���ʽΪ_______________________________________��
�˻����ﻹ�ɽ����Թ�ҵ��ˮ�е�CN������Ϊ̼���κͰ�����Ӧ�����ӷ���ʽΪ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ṥҵβ���ж�������ĺ�������0.05%(�������)ʱ�辭����������ŷš�ijУ��ȤС�����ⶨ���Ṥҵβ���ж�������ĺ������������·�����
��������ͼ��ʾ��ͼ������������B����ȷ����ͨ��β�����������β��ͨ��һ�������֪Ũ�ȵĵ�ˮ�вⶨSO2�ĺ�������ϴ��ƿC����Һ��ɫ��ʧʱ�������رջ���A��
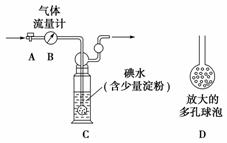
(1)ϴ��ƿC�е���ĩ������һ���������D���������ʵ���ȷ�ȣ��������ǣ�_________________________��
(2)ϴ��ƿC�е���Һ�����������Լ����������ٳ�һ�֣�___________________________________________��
(3)ϴ��ƿC����Һ��ɫ��ʧ��û�м�ʱ�رջ���A����õ�SO2����________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�ҷ�����ʵ�鲽������������ͼ��ʾ��

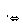
(4)д��������з�Ӧ�Ļ�ѧ����ʽ____________________________________________��
(5)�������ϴ�ӳ����ķ�����_________________________________________________��
(6)ͨ����β�����ΪV L(�ѻ���ɱ�״��)ʱ����β���ж�������ĺ���(�������)Ϊ________(�ú���V��m�Ĵ���ʽ��ʾ)��
�����������ҷ����в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba(OH)2��Һ�У����ಽ�����ҷ�����ͬ��
(7)����Ϊ�������Ƿ������˵�����ɣ�____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����̼���ƺ�̼�����ƹ���ķ����У���ͬ�����£����������
A�����ȣ��۲��Ƿ�������ų� B���μ�ϡ���ᣬ�Ƚϲ�������Ŀ���
C������ˮ��������ƣ��������� D�����Ⱥ�������������Ƿ�仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������̲��ŷḻ��Դ�������Ŀ������������ڻ��ǰ��Դ���ŵ����֣���ҵ�ϴӺ�ˮ����ȡ��ijЩ����ļ������̿�������ͼ��ʾ��
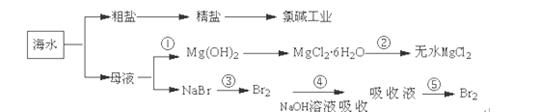
�����й�˵������ȷ����
A���ȼҵ�в��������ӽ���Ĥ���ۣ����Է�ֹ�������ķ�Ӧ
B���ڢڲ��нᾧ����MgCl2��6H2O���ܲ���ֱ���ڿ��������ȷֽ�����ˮMgCl2
C���ڢٲ���������ĸҺ�м�ŨNaOH��Һ����ȡMg(OH)2
D���ӵڢ۲����ڢݲ���Ŀ���ǻ�ú��嵥��Ũ�Ƚϸߵ���Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������Ļ��������뺬��23.4 g Na2O2���ܱ������У������������¶�Ϊ150 �棬�õ����ȼ��ַ�Ӧ��ѹǿΪ0 Pa����������������ˮ���������ݳ����ݴ��ж�����������ȷ����( )
A��ԭ��������м�������������Ϊ1��2 B��ԭ��������м�������������Ϊ2��1
C������������ֻ��NaOH D������������ֻ��Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
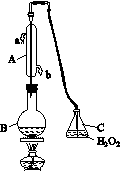
�л��������ұ�(CB2760��2011)�涨���Ѿ���SO2���ʹ����Ϊ0.25 g��L��1��ij��ȤС����ͼ(a)װ��(�г�װ����)�ռ�ij���Ѿ��е�SO2�������京�����вⶨ��
(a)
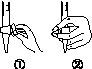 ��
��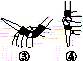
(b)
(1)����A��������__________________��ˮͨ��A�Ľ���Ϊ________��
(2)B�м���300.00 mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C�е�H2O2��ȫ��Ӧ���仯ѧ����ʽΪ________________________��
(3)��ȥC�й�����H2O2��Ȼ����0.090 0 mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ��ͼ(b)�е�________�����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ________������50 mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������(�����)________(�٣�10 mL���ڣ�40 mL����<10 mL����>40 mL)��
(4)�ζ����յ�ʱ������NaOH��Һ25.00 mL�������Ѿ���SO2����Ϊ________ g��L��1��
(5)�òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com