
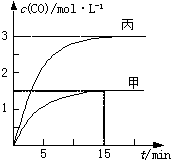
 һ�������´��ڷ�Ӧ��C��s��+H2O��g���TCO��g��+H2��g����H��0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��
һ�������´��ڷ�Ӧ��C��s��+H2O��g���TCO��g��+H2��g����H��0����ס��ҡ����������������м���һ����C��H2O�����������¶ȡ���Ӧ�����ʼ�����±�����Ӧ������CO�����ʵ���Ũ����ʱ��仯��ͼ��| ���� | �� | �� | �� |
| �ݻ� | 0.5L | 0.5L | V |
| �¶� | T1�� | T2�� | T1�� |
| ��ʼ�� | 2molC 1molH2O |
1molCO 1molH2 |
4molC 2molH2O |
| A���������У���Ӧ��ǰ15 min��ƽ������v��H2��=0.1 mol?L-1?min-1 |
| B�������������V��0.5 L |
| C�����¶�ΪT1��ʱ����Ӧ��ƽ�ⳣ��K=2.25 |
| D���������У���ƽ��ʱn��H2O��=0.4 mol����T1��T2 |
| ��c |
| ��t |
| c(CO)c(H2) |
| c(H2O) |
| 1.5��1.5 |
| 0.5 |
| 1.5mol/L |
| 15min |
| c(CO)c(H2) |
| c(H2O) |
| 1.5��1.5 |
| 0.5 |


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H+ |
| ��ת�� |
| Fe2+ |
| �ڻ�ԭ |
| OH- |
| �۳��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ס����У��淴Ӧ�����ʣ�v���ף�=v������ | B�������ס����У�HI �����ʵ�����n���ף���n���ң� | C�������ס������йط�Ӧ���ת���ʣ�����H2��+����HI����100% | D�������ס����У���ѧƽ�ⳣ����K���ף�=K���ң� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������Ӧ������ͬ | B���������з�Ӧ��ƽ�ⳣ����ͬ | C����������CO�����ʵ������������еĶ� | D����������CO��ת��������������CO2��ת����֮�͵���1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com