
����P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ��ͼ�еġ�H��ʾ����1mol��������ݣ���
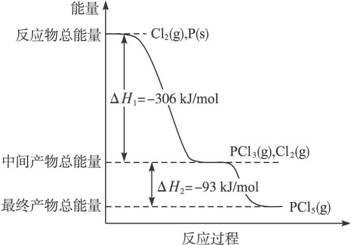
������ͼ�ش��������⣺
��1��P��Cl2��Ӧ����PCl3���Ȼ�ѧ���� ʽ��
ʽ��
��������������������������������������������������������������������������
��2��PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ��
��������������������������������������������������������������������������
�����ֽⷴӦ��һ�����淴Ӧ���¶�T1ʱ�����ܱ������м���0.80mol PCl5,��Ӧ��ƽ��ʱPCl5��ʣ0.60 mol����ֽ�����1������������������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ��2����2 ��1(����ڡ�����С�ڡ����ڡ�)��
��3����ҵ���Ʊ�PCl5ͨ�����������У��Ƚ�P��Cl2��Ӧ�����м����PCl3��Ȼ���£��ٺ�Cl2��Ӧ����PCl5��ԭ����________________________��������������������������������������������
��4��P��Cl2��������Ӧ����1mol PCl5�ġ�H3=____________��P��Cl2һ����Ӧ����1molPCl5�ġ�H4__________��H3(����ڡ�����С�ڡ����ڡ�)��
��5��PCl5������ˮ��ַ�Ӧ���������������ᣬ�仯ѧ����ʽ��__________________________________��
���𰸡�
��1��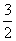 Cl2 (g)�� P(s)
Cl2 (g)�� P(s) PCl3 (g) ��H����306 kJ/ lmol������������������������
PCl3 (g) ��H����306 kJ/ lmol������������������������
��2�� PCl5(g) PCl3(g)��Cl2(g) ��H��93kJ/ mol����������������������������
PCl3(g)��Cl2(g) ��H��93kJ/ mol����������������������������
25�������������ڡ�������������������������������������������������
��3��������Ӧ��Ϊ���ȷ�Ӧ��������������߲��ʣ���ֹ����ֽ⡡����������
��4����399kJ/ mol������  ������
������
��5��PCl5��4H2O��H3PO4��5HCl
����������1����ͼ��֪P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ��P��s)+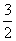 Cl2(g)
Cl2(g) PCl3(g) ��H=-306 kJ��mol-1��
PCl3(g) ��H=-306 kJ��mol-1��
��2����H=������������-��Ӧ������������ӦPCl3(g)+Cl2(g) PCl5(g) ��H=-93 kJ��mol-1���ʷ�ӦPCl5(g)
PCl5(g) ��H=-93 kJ��mol-1���ʷ�ӦPCl5(g) PCl3(g)+Cl2(g) ��H=+93 kJ��mol-1��
PCl3(g)+Cl2(g) ��H=+93 kJ��mol-1��
�ֽ���= ��100%=25%��
��100%=25%��
���ڷ�ӦPCl5 PCl3+Cl2�����ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ����ֽ���������2���ڦ�1��
PCl3+Cl2�����ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ�����ƶ����ֽ���������2���ڦ�1��
��3��3Cl2+2P 2PCl3��PCl3+Cl2
2PCl3��PCl3+Cl2 PCl5������Ӧ��Ϊ���ȷ�Ӧ�������¶ȣ�������ƽ���������ƶ��������PCl3��ת���ʣ�ͬʱ��ֹPCl5�ֽ⡣
PCl5������Ӧ��Ϊ���ȷ�Ӧ�������¶ȣ�������ƽ���������ƶ��������PCl3��ת���ʣ�ͬʱ��ֹPCl5�ֽ⡣
��4�����ݸ�˹���ɣ�P��Cl2��������Ӧ��һ����Ӧ����PCl5�Ħ�HӦ������ȵġ�
��5��PCl5��H2O��Ӧ���ɵ��������H3PO4��HCl��HClO����PCl5 H3PO4��PԪ�ػ��ϼ�û�䣬��ClԪ�ػ��ϼ�Ҳ���䣬���÷�Ӧ���ɵ���������H3PO4��HCl�����Ը÷�Ӧ�Ļ�ѧ����ʽ��PCl5+4H2O
H3PO4��PԪ�ػ��ϼ�û�䣬��ClԪ�ػ��ϼ�Ҳ���䣬���÷�Ӧ���ɵ���������H3PO4��HCl�����Ը÷�Ӧ�Ļ�ѧ����ʽ��PCl5+4H2O H3PO4+5HCl��
H3PO4+5HCl��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ڹ�������������Ӧ��ֻ����һ��һ�ȴ������ �� ��
A.CH3CH2CH2CH3 B.CH3CH(CH3)2
C.CH3C(CH3)3 D.(CH3)2CHCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и�����������Һ��һ���ܴ����������
A��pH=12����Һ��K+��Na+��CH3COO����CO32��
B��������Ӧ����������������Һ��Mg2+��K+��HCO3����NO3��
C����ˮ���������c(H+)=10-13mol/L����Һ��NH4+��Ca2+��SO32����Cl��
D��0.1 mol/L��NaNO3��Һ��H+��Fe2+��Cl����SO42��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��2H2(g)+O2(g)====2H2O(l) ��H=-571.6 kJ��mol-1
CH4(g)+2O2(g)====CO2(g)+2H2O(l) ��H=-890 kJ��mol-1
����H2��CH4�Ļ������112 L����״������ʹ����ȫȼ������CO2��H2O(l)����ʵ���÷�Ӧ����3 695 kJ����ԭ���������H2��CH4�����ʵ���֮����( )
A. 1��1 B.1��3 C.1��4 D.2��3
1��1 B.1��3 C.1��4 D.2��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼��������ֽ����������֯���Ƹ����ҵ����Ҫԭ�ϡ���ҵ̼���ƣ�����Լ98%���к���Ca2+��Mg2+��Fe3+��Cl-�� �����ʣ��ᴿ����·�����£�
�����ʣ��ᴿ����·�����£�
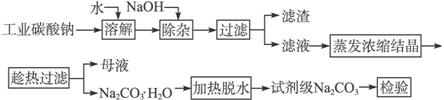
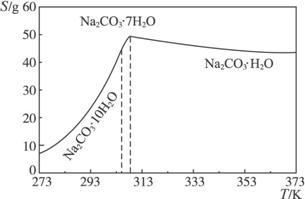
��֪̼���Ƶ��ܽ�ȣ�S�����¶ȱ仯����������ͼ��ʾ��
�ش��������⣺
��1����������Ҫ�ɷ�Ϊ________________��
��2�������ȹ��ˡ���ԭ����________________________________________��
��3������ʵ���ҽ��С����ȹ��ˡ����ɲ�ȡ�Ĵ�ʩ��_______________________��д��1�֣���
��4������ĸҺ��ѭ��ʹ�ã����ܳ��ֵ����⼰��ԭ����_____________________________��
��5����֪��Na2CO3��10H2O��s�� Na2CO3(s)+10H2O(g) ��H1=+532.36 kJ��mol-1
Na2CO3(s)+10H2O(g) ��H1=+532.36 kJ��mol-1
Na2CO3��10H2O��s�� Na2CO3��H2O��s��+9H2O(g) ��H2=+473.63 kJ��mol-1
Na2CO3��H2O��s��+9H2O(g) ��H2=+473.63 kJ��mol-1
д��Na2CO3��H2O��ˮ��Ӧ���Ȼ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪����1��Zn��s��+1/2O2��g��==ZnO(s)����H=-348.3kJ/mol
(2) 2Ag(s)+1/2 O2��g��== Ag2O(s)����H=-31.0kJ/mol
��Zn��s��+ Ag2O(s)== ZnO(s)+ 2Ag(s)�Ħ�H����
A.-317.3kJ/mol B. -379.3kJ/mol
-379.3kJ/mol
C.-332.8 kJ/mol D.317.3 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
(1)����������SO2����������SO3��
2SO2(s)+O2��g�� 2SO3��g��.
2SO3��g��.
ij�¶��£�SO2��ƽ��ת����(��)����ϵ��ѹǿ(P)�Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
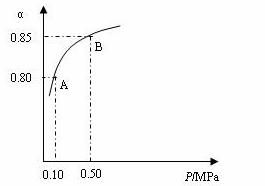
�ٽ�2.0 molSO2��1.0molO2����10 L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa���÷�Ӧ��ƽ�ⳣ������__________��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��_______K(B)(���������������=��)��
(2)��CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ��mol-1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ��mol-1
���ñ�״����4.48 L CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ__________(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ___________kJ��
(3)�������ײ�����ȱλ������(MFe2Ox��3��x��4��M=Mn��Co��Zn��Ni)��������(MFe2O4)�����»�ԭ���ã������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
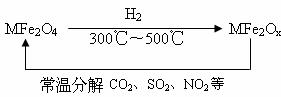
��д��MFe2Ox�ֽ�SO2�Ļ�ѧ����ʽ________________(������ƽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����ñ�����ⶨδ֪Ũ��NaOH��Һ���ü�����ָʾ�������в�������ʹ�ⶨ���ƫ�͵��ǣ�������
A����ʽ�ζ�����װ��Һǰδ�ñ���Һ��ϴ
B����ʼʱ��ʽ�ζ��ܼ��첿���������ݣ��ζ���������ʧ
C����ƿ����Һ��ɫ�ɻƱ��ʱ����ֹͣ�ζ�
D��ʢNaOH��Һ����ƿ�ζ�ǰ��NaOH��Һ��ϴ2��3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com