
ЮвЙњЕкЖўДњЩэЗнжЄВЩгУЕФЪЧОпгаТЬЩЋЛЗБЃадФмЕФPETGаТВФСЯЃЌPETGаТВФСЯПЩвдЛиЪедйРћгУЃЌЖјЧвЖджмБпЛЗОГВЛЙЙГЩШЮКЮЮлШОЁЃPETGЕФНсЙЙМђЪНШчЯТЃК

етжжВФСЯПЩВЩгУШчЯТЭМЫљЪОЕФКЯГЩТЗЯп

![]()
(2)RCOOR1+R2OH![]() RCOOR2+R1OH(RЁЂR1ЁЂR2БэЪОЬўЛљ)
RCOOR2+R1OH(RЁЂR1ЁЂR2БэЪОЬўЛљ)
ЪдЛиД№ЯТСаЮЪЬтЃК
(1)ЂпЕФЗДгІРраЭЪЧ__________________________________________ЁЃ
(2)аДГіIЕФНсЙЙМђЪНЃК_____________________________________________ЁЃ
(3)КЯГЩЪБгІПижЦЕФЕЅЬхЕФЮяжЪЕФСПЃКn(H)ЁУn(E)ЁУn(D)=____________(гУmЁЂnБэЪО)ЁЃ
(4)аДГіЗДгІЂкЕФЛЏбЇЗНГЬЪНЃК____________________________________ЁЃ
(5)аДГіЭЌЪБЗћКЯЯТСаСНЯювЊЧѓЕФEЕФЫљгаЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЁЃ
ЂйИУЭЌЗжвьЙЙЬхЕФБНЛЗЩЯЯрСкЕФШ§ИіЬМдзгЩЯЖМСЌгаШЁДњЛљЁЃ
ЂкИУЭЌЗжвьЙЙЬхдквЛЖЈЬѕМўЯТФмЗЂЩњвјОЕЗДгІКЭЫЎНтЗДгІЃЌгіЕНFeCl3ШмвКЯдзЯЩЋЁЃ
__________________ЁЂ_____________ЁЂ_____________ЁЃ
(1)ШЁДњЗДгІ
(2)![]()
(3)nЁУ(m+n)ЁУm
(4)CH2BrЁЊCH2Br+2H2O![]() CH2OHЁЊCH2OH+2HBr
CH2OHЁЊCH2OH+2HBr

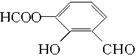
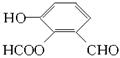
НтЮіЃКDЁЂEЁЂHОЙ§ЫѕОлЗДгІЩњГЩPETGЃЌPETGЫЎНтПЩвдЩњГЩШ§жжЮяжЪЃЌЗжБ№ЮЊHOCH2CH2OHЁЂ
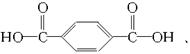
![]() ЁЃгЩЗДгІЬѕМўПЩжЊЃЌBЪЧH3CCH3ЃЌBгыТШЦјЗЂЩњШЁДњЗДгІЕУЃКFЃК
ЁЃгЩЗДгІЬѕМўПЩжЊЃЌBЪЧH3CCH3ЃЌBгыТШЦјЗЂЩњШЁДњЗДгІЕУЃКFЃК ![]() ЃЌЗДгІЂмЪЧЫЎНтЗДгІЃЌЪдМСXЪЧNaOHШмвКЃЌGЮяжЪЪЧCH2HOCH2OHЃЌGгыЧтЦјЗЂЩњМгГЩЗДгІЃЌЕУЕНHЮяжЪЃЌHЪЧ
ЃЌЗДгІЂмЪЧЫЎНтЗДгІЃЌЪдМСXЪЧNaOHШмвКЃЌGЮяжЪЪЧCH2HOCH2OHЃЌGгыЧтЦјЗЂЩњМгГЩЗДгІЃЌЕУЕНHЮяжЪЃЌHЪЧ![]() ЃЌЬўAЪЧCH2ЃЌЗЂЩњМгГЩЗДгІКѓЕУЕНCH2BrЁЊCH2BrЃЌИУТБДњЬўЫЎНтЕУЕНHOCH2CH2OHЃЌМДЮЊDЮяжЪЁЃ
ЃЌЬўAЪЧCH2ЃЌЗЂЩњМгГЩЗДгІКѓЕУЕНCH2BrЁЊCH2BrЃЌИУТБДњЬўЫЎНтЕУЕНHOCH2CH2OHЃЌМДЮЊDЮяжЪЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
вдТШЛЏФЦКЭСђЫсяЇЮЊдСЯжЦБИТШЛЏяЇМАИБВњЦЗСђЫсФЦЃЌЙЄвеСїГЬШчЯТЃК


ТШЛЏяЇКЭСђЫсФЦЕФШмНтЖШЫцЮТЖШБфЛЏШчЩЯЭМЫљЪОЁЃЛиД№ЯТСаЮЪЬтЃК
(1)гћжЦБИ10.7 g NH4ClЃЌРэТлЩЯашNaCl_______________gЁЃ
(2)ЪЕбщЪвНјааеєЗЂХЈЫѕгУЕНЕФжївЊвЧЦїга_______________ЁЂЩеБЁЂВЃСЇАєЁЂОЦОЋЕЦЕШЁЃ
(3)ЁАРфШДНсОЇЁБЙ§ГЬжаЃЌЮіГіNH4ClОЇЬхЕФКЯЪЪЮТЖШЮЊ_______________ЁЃ
(4)ВЛгУЦфЫћЪдМСЃЌМьВщNH4ClВњЦЗЪЧЗёДПОЛЕФЗНЗЈМАВйзїЪЧ_______________ЁЃ
(5)ШєNH4ClВњЦЗжаКЌгаСђЫсФЦдгжЪЃЌНјвЛВНЬсДПВњЦЗЕФЗНЗЈЪЧ_______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЯжгаВПЗжЖЬжмЦкдЊЫиЕФаджЪЛђдзгНсЙЙШчЯТБэЃК
дЊЫиБрКХ | дЊЫиаджЪЛђдзгНсЙЙ |
T | ЕЅжЪФмгыЫЎОчСвЗДгІЃЌЫљЕУШмвКГЪШѕЫсад |
X | LВуpЕчзгЪ§БШsЕчзгЪ§Жр2Иі |
Y | ЕкШ§жмЦкдЊЫиЕФМђЕЅРызгжаАыОЖзюаЁ |
Z | LВугаШ§ИіЮДГЩЖдЕчзг |
(1)аДГідЊЫиXЕФРызгНсЙЙЪОвтЭМ______________________________ЁЃ
аДГідЊЫиZЕФЕчзгХХВМЪН_____________________________________________ЁЃ
(2)аДГіYдЊЫизюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФЕчРыЗНГЬЪН_______________ЁЃ
(3)дЊЫиTгыТШдЊЫиЯрБШЃЌЗЧН№ЪєадНЯЧПЕФЪЧ_______________(гУдЊЫиЗћКХБэЪО)ЃЌЯТСаБэЪіжаФмжЄУїетвЛЪТЪЕЕФЪЧ_______________ЁЃ
a.ГЃЮТЯТТШЦјЕФбеЩЋБШTЕЅжЪЕФбеЩЋЩю
b.TЕФЕЅжЪЭЈШыТШЛЏФЦЫЎШмвКВЛФмжУЛЛГіТШЦј
c.ТШгыTаЮГЩЕФЛЏКЯЮяжаТШдЊЫиГЪе§МлЬЌ
(4)ЬНбАЮяжЪЕФаджЪВювьадЪЧбЇЯАЕФживЊЗНЗЈжЎвЛЁЃTЁЂXЁЂYЁЂZЫФжждЊЫиЕФЕЅжЪжаЛЏбЇаджЪУїЯдВЛЭЌгкЦфЫћШ§жжЕЅжЪЕФЪЧ_______________(гУдЊЫиЗћКХБэЪО)ЃЌРэгЩЪЧ__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com