
 2K2SO4+2Al2O3 +9SO2 +48H2O�������ж���ȷ����
2K2SO4+2Al2O3 +9SO2 +48H2O�������ж���ȷ����| A���ڱ��������ķ�Ӧ�У���ԭ���������������ʵ���֮����3��4 |
| B�����õ���K2SO4��Һ�����ԣ�����c(K+)=c(SO42-) |
| C�����ղ�����SO2������������,����948 t����(M=" 474" g/mol)����SO2��������Ϊ96%����������������Ϊ98%������432 t |
| D����ҵ��ұ��Al2O3�Ƶ�Al����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ط�Ӧ�Ļ�ѧ����ʽ�� |
 NaAlO2��3Ni(OH)2
NaAlO2��3Ni(OH)2 Ni(OH)2��OH������Ӽ��ܷ�ӦAl��3NiO(OH)��NaOH��H2O
Ni(OH)2��OH������Ӽ��ܷ�ӦAl��3NiO(OH)��NaOH��H2O 3Ni(OH)2��NaAlO2��
3Ni(OH)2��NaAlO2��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
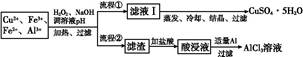
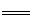
| ���� | Fe3+ | Fe2+ | Al3+ | Cu2+ |
| pH��Χ | 2.2��3.2 | 5.5��9.0 | 4.1��5.0 | 5.3��6.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

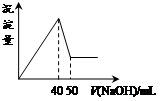
| A��1:3 | B��1:2 | C��1:1 | D��2:1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
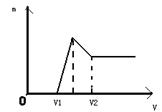
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
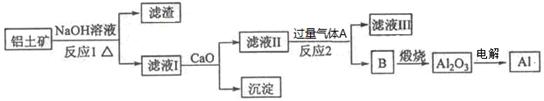
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
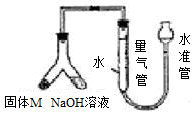
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��MnO2 | B��Fe��O�� | C��Cr2O3 | D��V2O5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
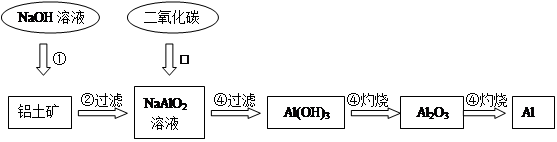
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com