
��ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g) == CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g) == CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g) == 2CO2(g) ��H3<0 ��
2H2(g)+O2 (g) == 2H2O(g) ��H4<0 ��
��ش��������⣺
��1�� ;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������
��2�� ��H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110��35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ���
��1������ �� ��2����H1=��H2+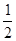 ����H3+��H4��
����H3+��H4��
��3��C(s) +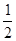 O2 (g) = CO(g) ��H=-110��35 kJ��mol-1
O2 (g) = CO(g) ��H=-110��35 kJ��mol-1
��4��ȼ��ȼ�ճ�֣������ʸߣ����ȶ࣬��ȾС��
��������������ɸ�˹����֪��Ӧ������;���أ�����;����ų�����������;����ų����������Ҳ���ȷ����H1����H2����H3����H4����ѧ��ϵʽ�ǡ�H1=��H2+1/2����H3+��H4��;
���㣺�Ȼ�ѧ����ʽ����д����˹���ɡ�


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д� Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ش��������⣺
����֪����CO(g) + 1/2O2(g) = CO2(g) ��H= ��283.0kJ��mol��1
��CH3OH(l) + 3/2O2(g) = CO2(g)+2H2O(l) ��H= ��726.5kJ��mol��1
��д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��____________________________________________________________________��
����֪��1molH��H����1molCl��Cl����1molH��Cl���ֱ���Ҫ��������436kJ��243kJ��432kJ����Ӧ��Ӧ��H2(g)+ Cl2(g)=2HCl (g) �ġ�H= ��
��2����֪25�桢101 kPa�£�ϡ��ǿ����ϡ��ǿ����Һ��Ӧ���к���Ϊ -57.3 kJ/mol��
�����ʾϡ������ϡ�ռ���Һ�кͷ�Ӧ���Ȼ�ѧ����ʽΪ�� ��
�ڲⶨ�к���ʵ��ʱ����IJ����������ձ�����Ͳ�� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ܼ��š�������ȫ�����������ŷţ�����ʮ���ش�����̼�IJ�������ʵ������������ŵ���Ҫ;��֮һ����ѧ������ ��Һ���ܡ����������е�
��Һ���ܡ����������е� ��
��
��1��ʹ�ù��� ��Һ����
��Һ���� ����Ӧ�����ӷ���ʽΪ________��������3molNaOH����Һ��������22��4L
����Ӧ�����ӷ���ʽΪ________��������3molNaOH����Һ��������22��4L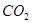 ���壨��״��������������Һ������̼Ԫ�ص������غ��ϵʽΪ__________��������Ũ�ȵĹ�ϵʽ��ʾ����
���壨��״��������������Һ������̼Ԫ�ص������غ��ϵʽΪ__________��������Ũ�ȵĹ�ϵʽ��ʾ����
��2������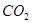 ��
��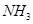 Ϊԭ�Ͽɺϳɻ�������[
Ϊԭ�Ͽɺϳɻ�������[ ]����֪��
]����֪��
 ��
��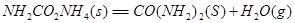
 ��
��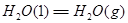
 ��
��
���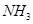 ��
��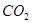 �ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ__________��
�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ__________��
��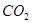 ͨ����Ӧ��ת��Ϊ
ͨ����Ӧ��ת��Ϊ ���ڴ���������CO��
���ڴ���������CO��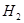 ��Ӧ���ɼ״���
��Ӧ���ɼ״��� ij�ݻ��ɱ���ܱ������г���10molCO��20mol
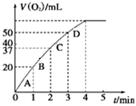
ij�ݻ��ɱ���ܱ������г���10molCO��20mol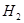 ��CO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
��CO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
A����A���ʾ��ijʱ�̴ﵽ��ƽ��״̬����ʱ�������ݻ�ΪVL������¶��µ�ƽ�ⳣ��K=__________��ƽ��״̬B��ʱ�������ݻ� _______VL��������ڡ�����С�ڡ����ڡ���
_______VL��������ڡ�����С�ڡ����ڡ���
B����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��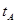 _______
_______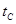 ���>������<����=����
���>������<����=����
C���ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��________��д��һ�ּ��ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪H2��g����CO��g����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-283.0kJ��mol��
��һ����̼��Һ̬ˮ��Ӧ�����ɶ�����̼���������Ȼ�ѧ����ʽΪ
________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣���ú��Ϊȼ�Ͽ�ͨ����������;����
;��I��C(s) +O2 (g)=CO2(g) ��H1<0 ��
;��II�����Ƴ�ˮú����C(s) +H2O(g)=CO(g)+H2(g) ��H2>0 ��
��ȼ��ˮú����2CO(g)+O2 (g)=2CO2(g) ��H3<0 ��
2H2(g)+O2 (g)=2H2O(g) ��H4<0 ��
��ش��������⣺
��1��;��I�ų������� ( ����ڡ������ڡ���С�ڡ�) ;��II�ų���������
��2����H1����H2����H3����H4����ѧ��ϵʽ�� ��
��3��12g̿���������в���ȫȼ������һ����̼���ų�110.35kJ���������Ȼ�ѧ����ʽΪ ��
��4��ú̿��Ϊȼ�ϲ���;��II���ŵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
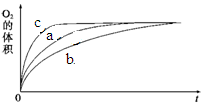
��1��ij��ѧ��ȤС��Ҫ��ɷ�Ӧ�ȵIJⶨ��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢��ͷ�ιܡ����β�����������0��50mol�� L��1���ᡢ0��50mol�� L��1NaOH��Һ��ʵ����ȱ�ٵIJ�����Ʒ��_____________��_______________��
��2����֪2molCO������ȫȼ������CO2 ����ų�566 kJ������1 mol������ȫȼ������Һ̬ˮ�ų�286 kJ������1 molCH4������ȫȼ������CO2�����Һ̬ˮ�ų�890 kJ������д���ñ�ȼ������Ϊ��Ӧ�ȵ�COȼ�յ��Ȼ�ѧ����ʽ__________________��
��1 molCH4������ȫȼ������CO2�����Һ̬ˮ���ų�����_____890 kJ�� ������� ����������=����������a molCH4��CO��H2�Ļ��������ȫȼ�գ����� CO2�����Һ̬ˮ����CO2��ˮ�����ʵ������ʱ����ų�������Q���ĵ�ȡֵ��Χ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ԫ���γɵij���������H2O��H2O2����һ�������¾��ɷֽ⡣
��1����֪��
| ��ѧ�� | �Ͽ�1mol��ѧ�������������kJ�� |
| H��H | 436 |
| O��H | 463 |
| O=O | 498 |
| ʵ���� | ��Ӧ�� | ���� | |
| a | 50 mL 5% H2O2��Һ | | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| b | 50 mL 5% H2O2��Һ | ����Ũ���� | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| c | 50 mL 5% H2O2��Һ | ����ŨNaOH��Һ | 1 mL 0.1 mol��L-1 FeCl3��Һ |
| d | 50 mL 5% H2O2��Һ | | MnO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������;�㷺����Ҫ��������Ӳ�ʻ����µĺϽ��Լ����ݵĵ�˿�������£����ܱ���������H2��ԭWO3�ɵõ������٣����ܷ�ӦΪ��
WO3 (s) + 3H2 (g) W (s) + 3H2O (g)
W (s) + 3H2O (g)
��ش��������⣺
��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___________________________��
��ij�¶��·�Ӧ��ƽ��ʱ��H2��ˮ�����������Ϊ2:3����H2��ƽ��ת����Ϊ_____________________�����¶ȵ����ߣ�H2��ˮ����������ȼ�С����÷�ӦΪ��Ӧ_____________________������ȡ����ȡ�����
�������ܷ�Ӧ���̴��·�Ϊ�����Σ�������Ҫ�ɷ����¶ȵĹ�ϵ���±���ʾ��
| �¶� | 25�� ~ 550�� ~ 600�� ~ 700�� |
| ��Ҫ�ɷ� | WO3 W2O5 WO2 W |
 W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1
W (s) + 2H2O (g)����H �� +66.0 kJ��mol��1  W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ��mol��1  WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________��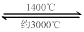 WI4 (g)������˵����ȷ����____________��
WI4 (g)������˵����ȷ����____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��1����֪��2Cu(s)��1/2O2(g)=Cu2O(s)����H = -169kJ��mol-1��
C(s)��1/2O2(g)=CO(g)����H = -110.5kJ��mol-1��
Cu(s)��1/2O2(g)=CuO(s)����H = -157kJ��mol-1
��̿���ڸ��������»�ԭCuO����Cu2O���Ȼ�ѧ����ʽ�ǣ�
��2����һ�������£���������������������·�Ӧ��2SO2(g)+O2(g) 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
��3���Լ��顢����Ϊ��Ӧ�KOH��Һ���������Һ����ȼ�ϵ�أ�����ӦʽΪ�� ��
��4����ˮAlCl3ƿ�Ǵ��а������䷴Ӧ�Ļ�ѧ����ʽΪ ��
��5����þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ���õ�ط�Ӧ���ܷ�Ӧ����ʽΪ_____________________��
��6����ҵ�ϵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com