
| A�� | 0.1mol•L-1��ˮ�У�c��OH-��=c��NH4+�� | |
| B�� | ��0.1mol•L-1CH3COONa��Һ�У�c��OH-��=c��CH3COOH��+c��H+�� | |
| C�� | 10 mL 0.02mol•L-1HCl��Һ��10 mL 0.02mol•L-1Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH=10 | |
| D�� | 0.1mol•L-1ij��Ԫ����ǿ����NaHA��Һ�У�c��Na+��=2c��A2-��+c��HA-��+c��H2A�� |
���� A����ˮ�д���ˮ�ĵ��롢һˮ�ϰ��ĵ��룻
B����������Һ�д��������غ㣻
C������Ϻ���Һ�����Ϊ20 mL��c��OH-��=$\frac{0.01L��0.02mol/L��2-0.01L��0.02mol/L}{0.02L}$=0.01mol/L��Kwδ֪�����ܼ���c��H+����
D��NaHA��Һn��Na��=n��A����
��� �⣺A����ˮ�д���ˮ�ĵ��롢һˮ�ϰ��ĵ��룬��0.1mol•L-1��ˮ�У�c��OH-����c��NH4+������A����
B����������Һ�д��������غ㣬��c��OH-��=c��CH3COOH��+c��H+������B��ȷ��
C������Ϻ���Һ�����Ϊ20 mL��c��OH-��=$\frac{0.01L��0.02mol/L��2-0.01L��0.02mol/L}{0.02L}$=0.01mol/L��Kwδ֪�����ܼ���c��H+��������ȷ��pH����C����
D��NaHA��Һn��Na��=n��A������c��Na+��=c��A2-��+c��HA-��+c��H2A������D����
��ѡB��
���� ���⿼������Ũ�ȴ�С�ıȽϣ�Ϊ��Ƶ���㣬���յ��롢ˮ�⼰�����غ㡢�����غ�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��ѡ��CΪ�״��㣬��Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 AsH3���ӵ�VSEPRģ������������ṹ�����幹������Ϊ���������еĹ��ۼ�Ϊ���Լ������Լ����Ǽ��Լ��������ڼ��Է��ӣ����Է��ӣ��Ǽ��Է��ӣ�����ԭ�Ӳ�ȡsp3�ӻ���
AsH3���ӵ�VSEPRģ������������ṹ�����幹������Ϊ���������еĹ��ۼ�Ϊ���Լ������Լ����Ǽ��Լ��������ڼ��Է��ӣ����Է��ӣ��Ǽ��Է��ӣ�����ԭ�Ӳ�ȡsp3�ӻ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ƕ������ȷ�Ӧ | B�� | a��b��c��Ϊ��ֵ | ||
| C�� | a=b | D�� | 2b=c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ϊ11��16 | B�� | �ܶȱ�Ϊ11��16 | C�� | �����Ϊ11��16 | D�� | ���Ӹ�����Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | �Ȼ��� | C�� | ��������Һ | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
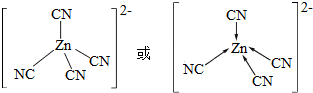 ��
��
�鿴�𰸺ͽ���>>
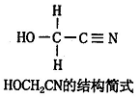
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
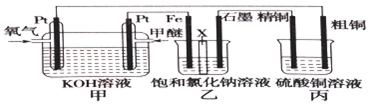
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ͬ��ԭ������SO2��CO��������� | |
| B�� | ���ʵ���Ũ��Ϊ1mol/L��NaOH��Һ��0.5mol/L��Ca��OH��2��Һ����n��OH-����� | |
| C�� | 1mol�Ҵ����ӵ�����Ϊ46g | |
| D�� | ���µ�ѹ�£�3mol C2H2��g����1mol C6H6��g�����ܶ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com