


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״��ϳɷ�ӦΪ��CO(g)+2H2(g) ![]() CH3OH(g) ��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
CH3OH(g) ��ҵ������Ȼ��Ϊԭ�ϣ���Ϊ�����Ʊ��״���
(1)�Ʊ��ϳ�����CH4+H2O��g��![]() CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO2+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м��������
CO+3H2��Ϊ����ϳ�����H2������CO��������⣬ԭ������������CO2��CO2+H2=CO+H2O��Ϊ��ʹ�ϳ��������ѣ�������ԭ�����м�������� ��̼�����Ϊ__________��
��̼�����Ϊ__________��
(2)�ϳɼ״����ٷ�Ӧ���������������仯����ͼ��ʾ��д���ϳɼ״����Ȼ�ѧ����ʽ__________________ ��
ʵ������1L�ܱ������н���ģ��ϳ�ʵ�顣��lmolCO��2molH2ͨ�������У��ֱ������300���500�淴Ӧ��ÿ��һ��ʱ���������м״���Ũ�����£�
 (�������ݵ�λ��mol��L��1)
(�������ݵ�λ��mol��L��1)
��300��ʱ��Ӧ��ʼ10�����ڣ�H2��ƽ����Ӧ����Ϊ__________��
��500��ʱƽ�ⳣ��K����ֵΪ___________��
��300��ʱ�����������ݻ�ѹ����ԭ����1��2���������������������£���ƽ����ϵ ������Ӱ����__________(����ĸ)��
a.c(H2)��С b������Ӧ���ʼӿ죬�淴Ӧ���ʼ���
c.CH3OH�����ʵ������� d������ƽ��ʱc(H2)��c(CH3OH)��С
 ��3���״�ֱ��ȼ�ջ����һ������Ⱦ��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�״�ȼ�ϵ�أ� �õ�ظ����ĵ缫��ӦʽΪ ������һ��ʱ���9��6g�״���ȫ��Ӧʱ���� NA������ת�ơ�
��3���״�ֱ��ȼ�ջ����һ������Ⱦ��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�״�ȼ�ϵ�أ� �õ�ظ����ĵ缫��ӦʽΪ ������һ��ʱ���9��6g�״���ȫ��Ӧʱ���� NA������ת�ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ĩ�� ���ͣ������
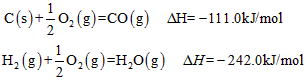
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com