
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣��ش��������⣺
��1������к͵ζ�������Ũ��Ϊ0.1000mol��L��1�ı�����ζ�δ֪Ũ�ȵ�NaOH��Һ�������м�¼��ʵ�����ݣ�
�ζ����� | ����Һ���(mL) | ���������(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.50 | 20.40 |
�ڶ��� | 20.00 | 3.00 | 23.00 |
������ | 20.00 | 4.00 | 24.10 |
�����в�����ɲⶨ���ƫ�ߵ���________(��ѡ����ĸ)
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ��ʢװ��Һ�ĵζ��ܼ��������ݣ��ζ���������ʧ
�ڸ�NaOH��Һ�����ʵ���Ũ��Ϊ_____________mol/L����С���������λ��Ч���֣�
��2��������ԭ�ζ�����ȡһ�����IJ��ᣨH2C2O4����Һ������ƿ�У���������ϡ���ᣬ�ñ����Ը��������Һ�ζ����ζ�ʱKMnO4��ҺӦװ��______________(��ᡱ�)ʽ�ζ����У��ζ��յ�ʱ�ζ�������_________________________________��
��3�������ζ��D�D�ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܡ��ο����е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����______(��ѡ����ĸ)��
������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | dz�� | �� | ש�� | �� |
Ksp | 1.77��10��10 | 5.35��10��13 | 1.21��10��16 | 1.12��10��12 | 1.0��10��12 |
A��NaClB��NaBrC��NaCND��Na2CrO4
���𰸡�CD 0.1000mol/L �� �������һ�α�Һ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ D
��������
(1)��A���ζ��յ����ʱ�����ӵζ��̶ܿȣ����¶�ȡ�ı�Һ���ƫС���ⶨ���ƫС�����������⣻
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ����Ӱ�����ĵı�Һ��������ԶԽ����Ӱ�죬���������⣻
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ������ϡ�ͱ�Һ�����¶�ȡ�ı�Һ���ƫ�ⶨ���ƫ�ߣ��������⣻
D���ζ�ǰ��ʢװ��Һ�ĵζ��ܼ��������ݣ��ζ���������ʧ�����ȡ�ı�Һ���ƫ�ⶨ���ƫ�������⣻
��������ѡCD��
�ڸ��ݱ���һ�����ı�Һ���Ϊ20.40mL-0.50mL=19.90mL���ڶ������ı�Һ���Ϊ23.00mL-3.00mL=20.00mL�����������ı�Һ���Ϊ24.10mL-4.00mL=20.10mL���������εζ����ĵ�HCl��Һƽ�����Ϊ20.00mL����NaOH��Һ�����ʵ���Ũ��Ϊc=![]() =0.1000mol/L��
=0.1000mol/L��
(2)���Ը��������Һ�����ԣ��Ҿ���ǿ�����ԣ�����װ����ʽ�ζ����У��ζ��յ�ʱ���Ը��������Һ��������Һ��Ϊ�Ϻ�ɫ����������Ϊ���������һ�α�Һ����ƿ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ�����ڲ���ɫ��
(3)����AgNO3ȥ�ζ�NaSCN��Һ����ѡ�õĵζ�ָʾ�������ʵ��ܽ��Ӧ��AgSCN��AgCl��AgBr��AgCN��AgSCN��Ϊͬ���ͳ�����ֻ��AgCl���ܶȻ���AgSCN��AgClͬΪ��ɫ�������������ԣ��������ֳ�����Ӧ���ξ������ʣ�Ag2CrO4��AgSCN�ܶȻ�����Ag2CrO4ΪA2B�ͳ���������Ag2CrO4���ܽ��Ҫ��AgSCN���ܽ��С����Ϊש��ɫ���������ԣ����Կ���ѡNa2CrO4��ָʾ������ѡD��


 ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�������ʵ������������������һ���ǣ� ��
ѡ�� | ʵ����������� | ʵ����� |
A | ��ij������Ʒ�м���Ba(OH)2��Һ���а�ɫ�������� | ����������һ����SO42- |
B | ��KI-������Һ�е�����ˮ����Һ�����ɫ | I-�Ļ�ԭ��ǿ��Cl- |
C | ��Ba(OH)2��8H2O��NH4Cl������С�ձ��л�Ͻ��裬���ִ����ձ���ڸо����� | Ba(OH)2��8H2O��NH4Cl�ķ�Ӧ�����ȷ�Ӧ |
D | ��ij����Һ�м���NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ���� | ������Һ�к���NH4+ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ����������������ء��밴Ҫ�ش��������⣺
��1������ĩ�ڹ��չ��顶�����ǡ��м����С�䳲����ķ����������ú���̼���Ƶ�ˮ��Һ��ϴ��˿������д��̼����ˮ��Һ��ͨ��CO2����Ļ�ѧ����ʽ____����54.8g Na2CO3��NaHCO3 �Ļ����ֳɵ��������ݣ�һ������ˮ������������ᣬ�ռ�������V L����һ��ֱ�Ӽ��������أ���������2.24L����������������ڱ�״���²ⶨ������ԭ����������Na2CO3�����ʵ�����n��Na2CO3����____������V��____��
��2����84������Һ��������ʹ�ù㷺������Ч�ɷ��Ǵ������ơ����ڳ����½�����ͨ��NaOH��Һ�Ƶã��÷�Ӧ�����ӷ���ʽΪ____������2mol��������÷�Ӧ�����ʱת�Ƶĵ�����Ϊ____NA��
��3��С�մ����������θ����࣬�䷴Ӧ�����ӷ���ʽΪ____��
��4����ʯ�ǵر���ʯ����Ҫ�����ҿ��ij�ֳ�ʯ�Ļ�ѧ���KAlSi3O8�����д��������������ʽΪ____��
��5��������(����ʽC6H12O6)�������ϸ����������Դ����֪1mol����1000mmol��ij��쵥��һЩָ����ͼ����ÿ������Ʒ�к������ǵ�����Ϊ____g���뱣����λС������
9 | ����� | 1.6 | |
10 | ��������ø | 161 | U/L |
11 | ���ἡ�ἤø | 56 | U/L |
12 | �������� | 0.52 | mmol/L |
13 | �ܵ��̴� | 4.27 | mmol/L |
14 | ���ܶ�֬�����̴� | 1.57 | mmol/L |
15 | ���ܶ�֬�����̴� | 1.40 | mmol/L |
16 | ������ | 4.94 | mmol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش��������⡣
I.����Ԫ�ص�ԭ�ӵ��Ӳ�ṹ���£�A��1s22s22p63s23p63d54s2��B��1s22s22p63s2��C��1s22s22p6��D��1s22s22p63s23p2��E��[Ar]4s1��
��ش�(��Ԫ�ط���)
��1��________Ԫ����ϡ�����塣��δ�ɶԵ���������Ԫ����________��
��2��AԪ��ԭ�ӵĺ�����ӹ���________���˶�״̬��������ߵ��ܼ���________�����ܼ����ţ���
��3��DԪ��ԭ�ӵļ۲�����Ų�ͼ��________��
��4��________Ԫ�صĵ縺�����________Ԫ��ԭ�ӵĵ�һ���������________Ԫ����������ɾ��д����ʵ������
II.Q��R��X��Y��Z����Ԫ�ص�ԭ���������ε�������Z���⣬����ľ�Ϊ����������Ԫ�ء���֪��
��Qԭ��2p�ܼ�����һ���չ����
��Rԭ�Ӻ���L�������Ϊ������
��Xԭ��2p�����ֻ��һ�������෴�ĵ��ӣ�
��Yԭ�Ӽ۵��ӣ���Χ���ӣ��Ų�msnmpn��
��Zԭ��M�����й��ȫ��������N���ɶԵ��ӣ�ֻ��1��δ�ɶԵ��ӡ���ش��������⣺
��5��Z2���ĺ�������Ų�ʽ��________��XԪ�ػ�̬ԭ�ӵĺ�������Ų�ͼ��____________��
��6��Q��Y�ֱ��γɵ������̬�⻯���У��ȶ��Ը�ǿ����________���ѧʽ����
��7��Q��R��X��Y����Ԫ�صĵ�һ��������ֵ�ɴ�С��˳��Ϊ________����Ԫ�ط������𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijǿ������ҺX�п��ܺ���Fe2+��Al3+��![]() ��
��![]() ��
��![]() ��
��![]() ��Cl���е������֣���ȡX��Һ��������ʵ�飬ʵ����̼�������ͼ������˵����ȷ���ǣ� ��
��Cl���е������֣���ȡX��Һ��������ʵ�飬ʵ����̼�������ͼ������˵����ȷ���ǣ� ��
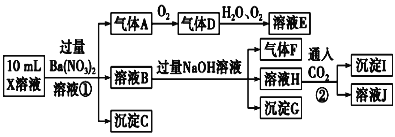
A.����A��NO2
B.X��Һ�п϶�����Fe2+��Al3+��![]() ��
��![]()
C.��ҺE������F���ܷ�����ѧ��Ӧ
D.X��Һ�в���ȷ����������Al3+��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Fe2����I����Br������Һ��ͨ��������������Һ�����������ӵ����ʵ����������������ʵ����ı仯����ͼ��ʾ������˵������ȷ����(����)
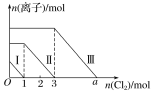
A.�߶�������Fe2���ı仯���
B.�߶�������Br���ı仯���
C.aֵ����6
D.ԭ�����Һ��n(FeBr2)��4mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����н�������A��B��C������ס��ҡ����Լ�����D��E��F��G��H������֮����ת����ϵ��ͼ��ʾ![]() ͼ����Щ��Ӧ��������ͷ�Ӧ������û�б��
ͼ����Щ��Ӧ��������ͷ�Ӧ������û�б��![]() ��
��
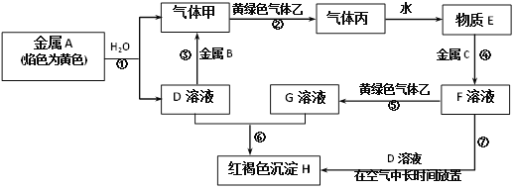
�����������Ϣ������и��⣺
(1)д���������ʵĻ�ѧʽ��B_______����__________��
(2)д������ɫ�����ҵ�һ����;___________����Ӧ���̢߿��ܹ۲쵽��ʵ��������______����Ӧ�Ļ�ѧ����ʽ��_______��
(3)��Ӧ���е����ӷ���ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��X����ѧ�����������������ͼת����ϵ������������ͷ�Ӧ������ȥ����
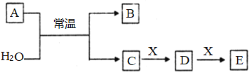
��1����AΪ�����Ľ������ʣ�������ɫ��Ӧ�ʻ�ɫ��X��ʹƷ����Һ��ɫ��д��C��E��Ӧ�����ӷ���ʽ��___________________________________________��
��2����AΪ������Ԫ����ɵĵ��ʣ���Ԫ�ص�����������ˮ����������ǿ����X����Ϊ__________������ĸ����
a. NaHCO3 b. Na2CO3 c.Al(OH)3 d.NaAlO2
��3����AΪ����ɫ��ĩ����A�ĵ���ʽΪ_______ ����XΪһ��������������ЧӦ�����塣������Ũ�ȵ�D��E������Һ����ѡ����Լ�Ϊ___��������ĸ��
a.���� b.CaCl2��Һ c.��ˮ d.����ʯ��ˮ
��4����AΪ�����X��Fe����ҺD�м���KSCN��Һ��졣��A��ˮ��Ӧ�Ļ�ѧ����ʽ���������뻹ԭ�������ʵ���֮��Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й���ͳ�Ļ��а�������Ƽ�֪ʶ�����й��������漰��ѧ�仯����
ǧ�����������࣬������ɳʼ���� |
������(CuSO4��5H2O) ��������֮�Ϊͭ |
��ʯ��(CaCO3)�� �������Ϊ�� |
��ɰ(HgS)��֮��ˮ���������ֳɵ�ɰ |
A | B | C | D |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com