�ڸ�һ��ѧ�̲��У������˹��������������̼��ˮ�ķ�Ӧ��
2Na
2O
2+2CO
2�T2Na
2CO
3+O
2 2Na
2O
2+2H
2O=4NaOH+O
2��
Ȼ�������������������ǽ���������磺NO��NO
2�ȣ��ķ�Ӧ�ܷ����أ�Ϊ�ˣ�ijУ��ѧ��ȤС�����������ʵ�����̽����
����һ��NO��NO
2���Ʊ�
�ٱ�ʵ�����õ�NO�ɱ�������������Һ��Ũ����ͷ�ĩ״����ط�Ӧ�Ʊ��������Ի�������������ӱ���ԭΪNO���õ���NO�Ĵ���ԼΪ98%��
��Ӧ�Ļ�ѧ����ʽΪ��
��NO
2������Ǧ���ȷֽ�õ���2Pb��NO
3��
2=2PbO+4NO
2��+O
2��ʹ���ɵ�����ͨ��
��������ѡ����ѡ����ѷ���������ʹNO
2���Ծ�����
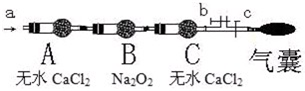
A��װ��H
2O��ϴ��ƿ B�����ڱ�ˮ�е�U�ιܣ�NO
2�е�21�棩
C��װ��Ũ�����ϴ��ƿ D��װ��Na
2SO
3��Һ��ϴ��ƿ
���������������ͼ��b��cΪ���ɼУ�
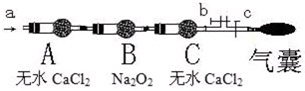
���ȴ�b�ر�c��ͨ��
��������ѡ��ѡ����5���ӣ�Ŀ����
A������ B��O
2C��CO
2 D��N
2��Ȼ��
���ٷֱ��a��ͨ��NO��NO
22���ӣ�
����ͨ��NOʱ��B�п���A��dz��ɫ�����ɻ�ɫ������δ�����𣻵�ͨ��NO
2ʱ��B�п���A��dz��ɫ�����ɰ�ɫ������Ҳδ������
���������������
�ֱ�ȡ��B�л�ɫ������ɫ������м��飬��֪���ֹ����Ϊ�Σ�
���ۣ�
���NO
2�� Na
2O
2��Ӧ�Ļ�ѧ����ʽ��
��



 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
