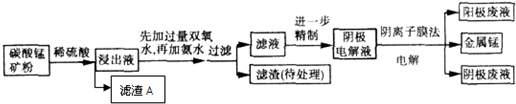
ij��̼���̿����Ҫ�ɷ���MnCO
3��MnO
2��FeCO
3��MgO��SiO
2��Al
2O
3�ȣ���֪̼����������ˮ��һ������������Ĥ��ⷨ���¼��������ڴ�̼���̿�����ȡ�����̣��������£�
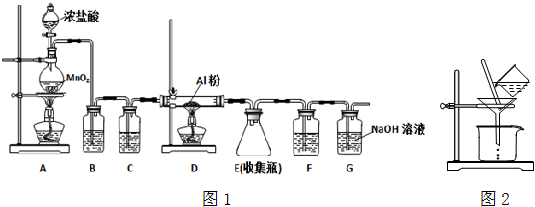
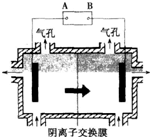
������Ĥ�����װ����ͼ��ʾ��
��1��д����ϡ�����ܽ�̼���̷�Ӧ�����ӷ���ʽ��
��
��2����֪Ksp ��Fe��OH��
3��=4��10
-38��������Һ��Fe
3+����Ũ��Ϊ0.1mol?L
-1����ʼ����Fe��OH��
3 ������pH��
��������ȫ��pH��
���������õ������ݣ�lg
=-0.1 lg
=0.2��
��3����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | A13+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 1.2 | 3.7 | 7.0 | 7.8 | 9.3 |
| ������ȫ��pH | 3.7 | 4.7 | 9.6 | 9.8 | 10.8 |
�Ӱ�ˮ������Һ��pH����6���������������������ijɷ���
��д��ѧʽ������Һ�к��е���������H
+��
��д���ţ���
��4���ڽ���Һ����Ԫ��ֻ��Mn
2+����ʽ���ڣ�������A��Ҳ��MnO
2���������ӷ���ʽ����ԭ��
��
��5�����װ���м�ͷ��ʾ��Һ���������ƶ��ķ�����A�缫��ֱ����Դ��
����ʵ�������У�������ϡ����Ϊ���Һ�������ĵ缫��ӦʽΪ
��
��6���ù���֮���Բ��������ӽ���Ĥ����Ϊ�˷�ֹMn
2+������������������Ӧ����MnO
2�����Դ�˷ѣ�д���ø���Ӧ�ĵ缫��Ӧʽ��
��