
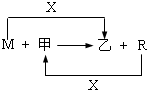
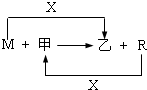
M��R���������г����Ľ������ʣ�����R���������Ľ������ס����ǻ�������м��Ǻ�ɫ���壬����R��x��ȼ�յõ���

(1)M����ڸ����·�Ӧ�Ļ�ѧ����ʽ��
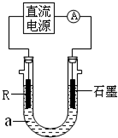
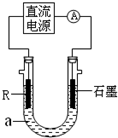
(2)��ⷨ��R�ĵͼ����������װ����ͼI��a��4 mol��L�� NaCl��1mol��L��NaOH�Ļ����Һ��
������aʱ��Ҫ��ȥ����ˮ���ܽ��O2�������� �ķ�����
��ʯī�缫Ӧ���Դ�� (���������)�������ӣ�ͨ���R�缫������������ ��
R���ĵ缫��Ӧʽ�� ��
��ֹͣʵ��һ��ʱ�����R���ϲ��к��ɫ���ʲ�������Ӧ�Ļ�ѧ����ʽ�� ��
(3)��R��ij�������ĩ��M��ĩ��Ϻ�ֳ����ȷݡ�һ���ڸ�����ǡ����ȫ��Ӧ�������������ᷴӦ����һ��ֱ�ӷ����������ռ���Һ�г�ַ�Ӧ��ǰ��������������ɵ�������������a��b����R��������Ļ�ѧʽ�� ��
(4)��ͼ��һ����ѧ���̵�ʾ��ͼ��
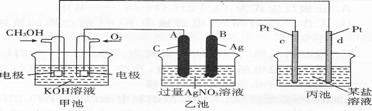
д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ
(5)���ҳ���B(Ag)������������5��40 g ʱ���׳�������������O2 ml
(��״����)����ʱ����ij�缫����1��60 gij����������е�ij����Һ������ (�����)��
A��MgSO4��Һ B��CuSO4��Һ C��NaCl��Һ D��AgN03��Һ
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������һģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��ɽ��ʡ�ij�������һ�и߿���ѧģ���Ծ����ţ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�걱�����������߿���ѧһģ�Ծ���4�·ݣ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com