

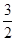 O2��g��=CO2��g����2H2O��l������H����725.76 kJ��mol��1��3.63��1014
O2��g��=CO2��g����2H2O��l������H����725.76 kJ��mol��1��3.63��1014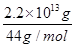 ��5��1011 mol���ų�����725.76 kJ��mol��1��5��1011 mol��3.63��1014 kJ����3������֪�Ȼ�ѧ����ʽ��Ŵ�ǰ����ֱ�Ϊ�٢ڢۣ��ۣ��ڣ��١�2���ɵ�����ʽ�����Ԧ�H����141 kJ��mol��1������566 kJ��mol��1��������393.5 kJ��mol��1����2����4������д������Ӧ�Ļ�ѧ����ʽ2O3��g��=3O2��g����ǰʽ��2����ʽ��3���ɵ������Ȼ�ѧ����ʽ��
��5��1011 mol���ų�����725.76 kJ��mol��1��5��1011 mol��3.63��1014 kJ����3������֪�Ȼ�ѧ����ʽ��Ŵ�ǰ����ֱ�Ϊ�٢ڢۣ��ۣ��ڣ��١�2���ɵ�����ʽ�����Ԧ�H����141 kJ��mol��1������566 kJ��mol��1��������393.5 kJ��mol��1����2����4������д������Ӧ�Ļ�ѧ����ʽ2O3��g��=3O2��g����ǰʽ��2����ʽ��3���ɵ������Ȼ�ѧ����ʽ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g) +H2O(g) ��H =��49.0 kJ��mol��1
CH3OH(g) +H2O(g) ��H =��49.0 kJ��mol��1 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2SO3(g)��H="-197" kJ��mol-1��ʵ����4 mol SO2�μ�������Ӧ�ų�354 kJ��������SO2��ת������ӽ���( )
2SO3(g)��H="-197" kJ��mol-1��ʵ����4 mol SO2�μ�������Ӧ�ų�354 kJ��������SO2��ת������ӽ���( )| A��90% | B��80% | C��50% | D��40% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| �ܽ��(S)/g | �ܶȻ�(Ksp) | ||
| Ca(OH)2 | Ba(OH)2 | CaSO3 | BaSO3 |
| 0.160 | 3.89 | 6.76��10��3 | 5.48��10��9 |
 ��____________(д������ʽ����)��
��____________(д������ʽ����)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)
O2(g) H2O(l)��H="-285.8" kJ/mol
H2O(l)��H="-285.8" kJ/mol CO2(g)+2H2O(l)��H="-890.3" kJ/mol
CO2(g)+2H2O(l)��H="-890.3" kJ/mol| A��1��1 | B��1��3 | C��1��4 | D��2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����69.4 kJ��mol��1�������� | B����45.2 kJ��mol��1 |
| C����69.4 kJ��mol��1 | D����45.2 kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ��
CO2(g) +3 H2 (g)�����CO2��CH3OH(g)��Ũ����ʱ��仯���±���ʾ�� | ʱ�� ���� | 0 min | 10 min | 30 min | 60 min | 70 min |
| CO2(mol/L) | 0 | 0.2 | 0.6 | 0.8 | 0.8 |
| CH3OH(mol/L) | 1.0 | 0.8 | 0.4 | 0.2 | 0.2 |
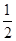 O2 (g)
O2 (g) CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol
CO2(g) + 2H2 (g) ?H1= ��192.9kJ/mol 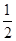 O2 (g)
O2 (g) H2 O(g) ?H2= ��120.9kJ/mol
H2 O(g) ?H2= ��120.9kJ/mol 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CH3OH(g)����H����90.1 kJ��mol��1 ��һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)����H����90.1 kJ��mol��1 ��һ��ѹǿ�£��ݻ�ΪV L�������г���a mol CO��2a mol H2���ڴ��������·�Ӧ���ɼ״���ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��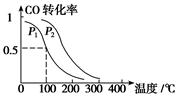

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2N (g)
2N (g) 2H (g)
2H (g)
 ��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ��
��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com