
��15��)ij�о���ѧϰС�齫һ��Ũ��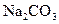 ��Һ����
��Һ���� ��Һ�еõ���ɫ������
��Һ�еõ���ɫ������
��ͬѧ��Ϊ���߷�Ӧ����ֻ�� һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ��������ٽ�ˮ�ⷴӦ������ һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ���� ��
�� ���ֳ�����
���ֳ�����
(��������֪�� ��
�� �������ᾧˮ)
�������ᾧˮ)
I��������ͬѧ������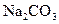 ��Һ��
��Һ�� ��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ�ٹ��ˢ�ϴ�Ӣ۸��
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
(1)��װ������˳��Ϊ �� �� ��
(2)װ��C��װ���Լ��������� ��
(3)��֤������������ ��ʵ�������� ��
��ʵ�������� ��
���� ��
�� ���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�
���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�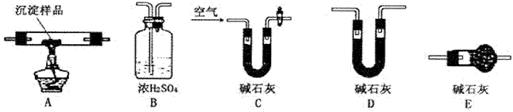
(1)װ��C�м�ʯ�ҵ������� ��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ŀ��������÷ֱ��� ��
(2)��������Ʒ������Ϊm�ˣ�װ��B����������n�ˣ�������� ����������Ϊ�� ��
������������ ��
��Na2CO3 +CuSO4 +H2O=Cu(OH)2��+Na2SO4+CO2����2�֣���
��1��A��C��B��2�֣���2����ˮ����ͭ��2�֣���3��װ��B�г���ʯ��ˮ����ǣ�2�֣�
��1�����տ����е�H2O ������CO2��2�֣�����ʼʱͨ�봦�����Ŀ������Խ�װ����ԭ�к�H2O ������CO2�Ŀ����ų�������ʱͨ�봦�����Ŀ������Խ�װ����������H2O ������CO2�ų�����2�֣�
����2��1����49n/9m����3�֣�
����


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��) ij�о���ѧϰС��Ϊȷ��ij��ʽ̼��þ��Ʒ����ɣ���Ƴ�����ͼ��ʾ��ʵ��װ�ã�ͼ��A��D�IJ��֣���[��֪��ʽ̼��þMgx(OH)y(CO3)z(x��y��zΪ�������������ֽܷ���������þ��ˮ�Ͷ�����̼]
��1������ͼ���г�����δ��������װ��ʵ��װ�ú�Ӧ���Ƚ��еIJ�����_____________��A�������ʢ�ŵ�ҩƷ��_____________����������____________________________��
��2��ָ����ʦ��������Ʒ�����ָ����Ҫ��E�����иĽ������������С����Ƴ��Ľ�������____________________________________��
��3������ǰ��Ҫ���еı�Ҫ������__________________________________����Ŀ����____________________________________���Ի����IJ��������ǣ��رջ��� ________������________��
��4��ͨ��ʲô�����ܹ�˵����ʽ̼��þ�ֽ���ȫ____________________________��
��5����Bװ���еķ�Ӧ��ȫ��K1���ٻ���������������ӣ���Ŀ����________________________��
��6��ʵ�����������£���ʽ̼��þ��Ʒ22.6 g����ӦǰCװ�õ�����Ϊ87.6 g����Ӧ������Ϊ89.4 g����ӦǰDװ�õ�����Ϊ74.7 g����Ӧ������Ϊ83.5 g��������Ƶ��ü�ʽ̼��þ�Ļ�ѧʽ___________________________,�ü�ʽ̼��þ���ȷֽ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(15��) ij�о���ѧϰС��Ϊȷ��ij��ʽ̼��þ��Ʒ����ɣ���Ƴ�����ͼ��ʾ��ʵ��װ�ã�ͼ��A��D�IJ��֣���[��֪��ʽ̼��þMgx(OH)y(CO3)z(x��y��zΪ�������������ֽܷ���������þ��ˮ�Ͷ�����̼]

��1������ͼ���г�����δ��������װ��ʵ��װ�ú�Ӧ���Ƚ��еIJ�����_____________��A�������ʢ�ŵ�ҩƷ��_____________����������____________________________��
��2��ָ����ʦ��������Ʒ�����ָ����Ҫ��E�����иĽ������������С����Ƴ��Ľ�������____________________________________��
��3������ǰ��Ҫ���еı�Ҫ������__________________________________����Ŀ����____________________________________���Ի����IJ��������ǣ��رջ��� ________������________��
��4��ͨ��ʲô�����ܹ�˵����ʽ̼��þ�ֽ���ȫ____________________________��
��5����Bװ���еķ�Ӧ��ȫ��K1���ٻ���������������ӣ���Ŀ����________________________��
��6��ʵ�����������£���ʽ̼��þ��Ʒ22.6 g����ӦǰCװ�õ�����Ϊ87.6 g����Ӧ������Ϊ89.4 g����ӦǰDװ�õ�����Ϊ74.7 g����Ӧ������Ϊ83.5 g��������Ƶ��ü�ʽ̼��þ�Ļ�ѧʽ___________________________,�ü�ʽ̼��þ���ȷֽ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15��)ij�о���ѧϰС�齫һ��Ũ��![]() ��Һ����
��Һ����![]() ��Һ�еõ���ɫ������
��Һ�еõ���ɫ������
��ͬѧ��Ϊ���߷�Ӧ����ֻ��![]() һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ��������ٽ�ˮ�ⷴӦ������![]() һ�ֳ�����
һ�ֳ�����
��ͬѧ��Ϊ����![]() ��
��![]() ���ֳ�����
���ֳ�����
(��������֪��![]() ��
��![]() �������ᾧˮ)
�������ᾧˮ)
I��������ͬѧ������![]() ��Һ��
��Һ��![]() ��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��Һ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ�ٹ��� ��ϴ�� �۸��
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�

(1)��װ������˳��Ϊ �� �� ��
(2)װ��C��װ���Լ��������� ��
(3)��֤������������![]() ��ʵ�������� ��
��ʵ�������� ��
����![]() ��
��![]() ���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�
���߶��У���ͨ��������ʾװ�õ����ӣ����ж����������ⶨ����ɡ�

(1)װ��C�м�ʯ�ҵ������� ��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ŀ��������÷ֱ��� ��
(2)��������Ʒ������Ϊm�ˣ�װ��B����������n�ˣ��������![]() ����������Ϊ�� ��
������������ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com