
| V |
| Vm |
| ���ʵ����� |
| ��Һ������ |
| 1000��w |
| M |
| 22.4L |
| 22.4L/mol |
| 36.5g |
| 36.5g+63.5g |
| 1000��1.18��36.5% |
| 36.5 |
| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | ʵ������ | ���ۼ����� |
| ��1��ȡ��������������������ˮ�У�����֧�Թֱܷ�ȡ������Һ�������ò�˿պȡ�Թ�1����Һ���� | �� | ���ۣ�����K+�� |
| ��2�����Թ�2�м������������ữ���μ����� | �а�ɫ������ | ���ۣ����� ���ͣ� ��д���ӷ���ʽ�� |
| ��3�����Թ�3����μ�������NaOH��Һ���ӱ��� | ���ۣ����� ���ͣ� ��д���ӷ���ʽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
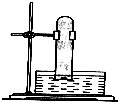 ij�о�С��Ϊ��̽�������������Ӧ�����������˼���ʵ�飮����д���пհף�
ij�о�С��Ϊ��̽�������������Ӧ�����������˼���ʵ�飮����д���пհף��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
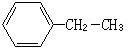
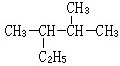
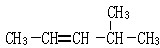
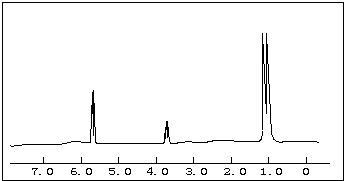
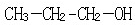 ��2-������
��2-������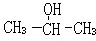 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ܢۢ� | B���ܢۢڢ� |
| C���ڢۢܢ� | D���٢ܢڢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com