
����һ����β��ͨ�백ˮ����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣��֪SO2����NH4��2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200 �棬���ⶨ������NH4��2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ50.00 mL��������120 �����ң�ʹ����ȫ���ݳ�������й�ʵ���������£�
ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
�� | 7.24 | 50.00 | 1.792 |
�� | 14.48 | 50.00 | 3.584 |
�� | 21.72 | 50.00 | 4.032 |
�� | 28.96 | 50.00 | 3.136 |
��1����ȡ3.62 g��Ʒ��ͬ������ʵ��ʱ�����ɰ������������״����Ϊ_________L��
��2���û�����У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ_________��
��3��������NaOH��Һ�����ʵ���Ũ��_________��
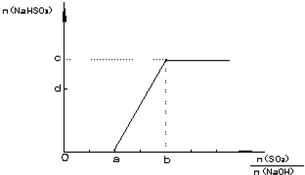
����������NaOH��Һ��ʯ�Ҽ�O2����β��������ȡʯ�ࣨCaSO4��2H2O�����˹��̵��м������NaHSO3��ʵ�ʲ������Ե���β���ŷŵ�������ȡ��SO2��NaOH�����ʵ�������ѱ�ֵ���Ӷ�������������ƵIJ�������д��n��SO2��/n��NaOH���ڲ�ͬȡֵ��Χʱ��n��NaHSO3����ֵ��n��NaHSO3����n��SO2����n��NaOH���Ĺ�ϵʽ�������±���
n��SO2��/n��NaOH�� | n��NaHSO3�� |
|
|
|
|
|
|
��1��0.896 ��2��1��2 (3)6 mol��L-1
�б����£�
n��SO2��/n��NaOH�� | n��NaHSO3�� |
��0.5 | 0 |
0.5��1 | n��NaHSO3��=2n(SO2)-n��NaOH�� |
��1 | n��NaHSO3��=n��NaOH�� |
��������1����ʵ��٢�֪NaOH������ȡ3.62 g��ƷʱҲ��NaOH��������������Ϊ![]() =0.896 L��
=0.896 L��
��2����7.24 g��Ʒ�У���NH4��2SO4���ʵ���Ϊx��NH4HSO4���ʵ���Ϊy����

���![]()
![]() ��
��
��3���Եڢ������ݼ��㣬��Ʒ������NaOH���㣬NaOH����H+�к�����![]() ��Ӧ����NH3����Һ������
��Ӧ����NH3����Һ������![]() ������n��NaOH��=n��NH4HSO4��+n��NH3����
������n��NaOH��=n��NH4HSO4��+n��NH3����
c��NaOH��= =6.00 mol��L-1��
=6.00 mol��L-1��
����������![]() ��0.5ʱ��NaOH������ȫ������Na2SO3����NaHSO3��
��0.5ʱ��NaOH������ȫ������Na2SO3����NaHSO3��
��![]() ��1ʱ��SO2������ȫ������NaHSO4����Na2SO3����0.5��
��1ʱ��SO2������ȫ������NaHSO4����Na2SO3����0.5��![]() ��1ʱ��SO2��NaOHȫ����Ӧ������ΪNa2SO3��NaHSO3�Ļ���
��1ʱ��SO2��NaOHȫ����Ӧ������ΪNa2SO3��NaHSO3�Ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L����״���� |
| 1 | 7.24 | 50.00 | 1.792 |
| 2 | 14.48 | 50.00 | 3.584 |
| 3 | 21.72 | 50.00 | 4.032 |
| 4 | 36.20 | 50.00 | 2.240 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ����β��ͨ�백ˮ����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2��(NH4)2SO4��NH4HSO4���塣��֪(NH4)2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200 �棬���ⶨ����(NH4)2SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ50.00 mL��������120 �����ң�ʹ����ȫ���ݳ�������й�ʵ���������£�
ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L(��״��) |
�� | 7.24 | 50.0 | 1.792 |
�� | 14.48 | 50.00 | 3.584 |
�� | 21.72 | 50.00 | 4.032 |
�� | 28.96 | 50.00 | 3.136 |
(1)��ȡ3.62 g��Ʒ��ͬ�ַ���ʵ��ʱ�����ɰ��������(��״��)Ϊ__________L��
(2)�û������(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ__________��
(3)������NaOH��Һ�����ʵ���Ũ��(д���������)��
����������NaOH��Һ��ʯ�Ҽ�O2����β��SO2������ȡʯ��(CaSO4��2H2O)���˹��̵��м������NaHSO3��ʵ�ʲ������Ե���β���ŷŵ�������ȡ��SO2��NaOH�����ʵ�������ѱ�ֵ���Ӷ�������������ƵIJ�������д��n(SO2)/n(NaOH)�ڲ�ͬȡֵ��Χʱ��n(NaHSO3)��ֵ��n(NaHSO3)��n(SO2)��n(NaOH)�Ĺ�ϵʽ�������±���
n(SO2)/n(NaOH) | n(NaHSO3) |
|
|
|
|
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�� | �����Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
1 | 3.62 | 50.00 | 0.896 |
2 | 7.24 | 50.00 | 1.792 |
3 | 10.86 | 50.00 | 2.016 |
4 | 14.48 | 50.00 | 1.568 |
(1)��1������ֱ���Ʋ⣬1.81 g��Ʒ����ͬ��ʵ��ʱ�����ɰ���������������Ϊ________ L��
��2���Լ���û�����У�NH4��2SO4��NH4HSO4�����ʵ���֮��Ϊ___________��
��3��������NaOH��Һ�����ʵ���Ũ��Ϊ___________ mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������ʮ���и�����ѧ�ڵڶ����¿���ѧ�Ծ����������� ���ͣ�������
��12�֣��Ӵ������������Ҫԭ����������Ϳ�����
I���Ӵ���������Ĺ����У���������Ҫ���豸�ǣ� �� ����������Ϊ�˱����������γɣ����SO3�������ʣ���ҵ�ϳ��� �������ռ���
II��Ϊ�˷�ֹ������Ⱦ����β�������ۺ����ã����᳧���ð�ˮ����β���е�SO2��SO3�����壬��������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣Ϊ�˲ⶨ������NH4��2SO4 ��NH4HSO4 �����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ����Ũ��Ϊ3.00 mol��L-1��NaOH��Һ50.00 mL��������120 �����ң�ʹ����ȫ���ݳ�����NH4��2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200 �桳������й�ʵ���������£���״������
| ʵ����� | ��Ʒ������/g | NaOH��Һ�����/mL | ���������/L |
| 1 | 3.62 | 50.00 | 0.896 |
| 2 | 7.24 | 50.00 | 1.792 |
| 3 | 10.86 | 50.00 | 2.016 |
| 4 | 14.48 | 50.00 | 1.568 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com