
���� ��1��ͬ��ͬѹ�£������NO��NO2�����ʵ�����ȣ���ͬ������������ܶ���Ħ�����������ȣ�
��2����ͬ������������ܶ�����Է������������ȣ�
��3��ͬ��ͬѹ�������ѹǿ�����ʵ��������ȣ�
��4���������ƹ�����������������ʵ�������������Ҫ2mol/L��������Һ�����
��5������n=$\frac{m}{M}$����������ӵ����ʵ������ٸ����������Ļ�ѧ����������������ӵ����ʵ�����������c=$\frac{n}{V}$���������Һ����������ӵ�Ũ�ȣ�
��6�����ƹ�������������ʵ������䣬�����ҪŨ��������Ϊx��Ȼ�����������غ���ʽ���㣻
��7������n=$\frac{V}{{V}_{m}}$�����Ȼ�������ʵ������ٸ���m=nM�����Ȼ�����������Һ������=1000g+m��HCl��������ܶ������Һ��������������ʵ���Ũ�ȹ�ʽc=$\frac{n}{V}$������Һ�����ʵ���Ũ��
��� �⣺��1��ͬ��ͬѹ�£��������NO��NO2�����ʵ�����ȣ������ʵ���֮��Ϊ1��1��
��ͬ����������������ܶ�֮�ȵ�����Ħ������֮��=30g/mol��46g/mol=15��23��
�ʴ�Ϊ��1��1��15��23��
��2��ij�������ܶ���ͬ��ͬѹ�������ܶȵ�15���������ܶ�����Է������������ȿ�֪�����ʵ���Է��������ǣ�2��15=30��
�ʴ�Ϊ��30��
��3��ͬ��ͬ����£������ѹǿ�����ʵ��������ȣ���1mol������3mol������̼��ѹǿ֮��Ϊ1mol��3mol=1��3��
�ʴ�Ϊ��1��3��
��4������100mL 0.5mol/L������Һ�����ƹ�������������ʵ������䣬����Ҫ2mol/L��������Һ���Ϊ��$\frac{0.5mol/L��0.1L}{2mol/L}$=0.025L=25mL��
�ʴ�Ϊ��25mL��
��5��VL Al2��SO4��3�к�Al3+mg�����������ӵ����ʵ���Ϊ��$\frac{mg}{27g/mol}$=$\frac{m}{27}$mol�����ݻ�ѧʽAl2��SO4��3��֪����Һ�к�����������ӵ����ʵ���Ϊ��$\frac{m}{27}$mol��$\frac{3}{2}$=$\frac{m}{18}$mol�������Һ��SO42-�����ʵ���Ũ��Ϊ��c��SO42-��=$\frac{\frac{m}{18}mol}{VL}$=$\frac{m}{18V}$mol/L��
�ʴ�Ϊ��$\frac{m}{18V}$��
��6����98%��ŨH2SO4����=1.84g•cm-3�����Ƴ�0.5mol/L��ϡH2SO4 500ml������ҪŨ��������ΪxmL���������ƹ�������������������֪��1.84g•cm-3��xmL��98%=98g/mol��0.5mol/L��0.5L����ã�x=13.6��
�ʴ�Ϊ��13.6��
��7������״���µ�V L HCl�����������ʵ���Ϊ$\frac{VL}{22.4L/mol}$=$\frac{V}{22.4}$mol���Ȼ��������Ϊ��$\frac{V}{22.4}$mol��36.5g/mol=$\frac{36.5V}{22.4}$g��
������Һ����Ϊ��1000g+$\frac{36.5V}{22.4}$g=��1000+$\frac{36.5V}{22.4}$��g��
������Һ�����Ϊ��V=$\frac{m}{��}$=$\frac{22400+36.5V}{22.4��}$mL=$\frac{22400+36.5V}{22.4��}$��10-3L��
������ҺŨ��Ϊ��$\frac{\frac{V}{22.4}mol}{\frac{22400+36.5V}{22.4�ѡ�1{0}^{-3}}L}$=$\frac{1000��V}{22400+36.5V}$mol/L��
�ʴ�Ϊ��$\frac{1000��V}{22400+36.5V}$mol/L��
���� ���⿼�������ʵ�����Ũ�ȡ�����Ħ��������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ���ʵ�����Ħ������������Ħ����������ʵ���Ũ�ȵ�֮��Ĺ�ϵΪ���ؼ�������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������ѧ����������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 150mL 1mol•L-1�Ȼ�����Һ | B�� | 75mL 1.5mol•L-1�Ȼ�����Һ | ||
| C�� | 150mL 3mol•L-1�������Һ | D�� | 50mL 1mol•L-1�Ȼ�þ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2CO3+HNO3��NaHCO3+HNO3 | B�� | CaCl2+Na2CO3��Ca��NO3��2+K2CO3 | ||
| C�� | Ba��OH��2+NaHSO4��BaCl2+NaHSO4 | D�� | NaOH+H2SO4��Fe��OH��3+H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
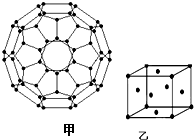 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �õιܵμ�Һ��ʱ���ι�Ӧ�����������Ϸ������ܴ��������ڱ� | |
| B�� | ��ҩ����ֽ�۰ѷ�ĩ״ҩƷ�����Թܵĵײ� | |
| C�� | ����������Һʱ����������Ͳ�м���һ�������ˮ�����ڽ�����������������Ũ���� | |
| D�� | ����ʱ��������ĩ��Ӧ������������ֽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
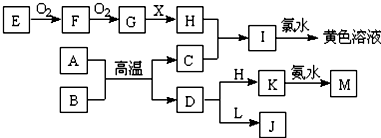
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
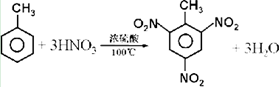 ��
��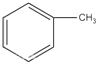 +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$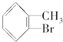 +HBr��
+HBr�� +Br2$\stackrel{Fe}{��}$
+Br2$\stackrel{Fe}{��}$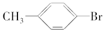 +HBr��
+HBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
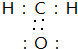
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com