�⣺��1���ٶ�����̼���������ڿ�����Ⱦ�������������NO
2��SO
2��Ϊ��Ⱦ���Ҫ��⣬�ʴ�Ϊ��D��
��A������̫���ܡ���ϫ�ܡ��������磬�Ի�ȡ�����Դ�������ڸ��ƻ�����������A��ȷ��
B�����ϵ�������������������ˮ����Ⱦ����B����
C�����ع��ͻ����ټӹ�Ϊʳ���ͣ�ֱ��Ӱ������Ľ�������C����
D�����ö�����̼��ԭ�Ϻϳɾ�̼������ɽ������ϴ������ϩ�����ٰ�ɫ��Ⱦ����D��ȷ��
�ʴ�Ϊ��AD��
��2������ú�м���ʯ��ʯ��Ϊ����������Լ���SO
2���ŷţ�����CaSO
4��
�����Ļ�ѧ��ӦΪ2CaCO
3+O
2+2SO
2
2CaSO
4+2CO
2��
�ʴ�Ϊ��2CaCO
3+O
2+2SO
2
2CaSO
4+2CO
2��
��NO��COת��Ϊ�����壬���ɵ����Ͷ�����̼���÷�ӦΪ2NO+2CO
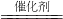
N
2+2CO
2��
�ʴ�Ϊ��2NO+2CO
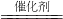
N
2+2CO
2��
��������������ˮ�����ɽ��壬���������Կɾ���ˮ����ҵ��ˮ�к��е�Cr
3+���ӣ�������ʯ������������
��pHΪ8��9ʱ���ɳ�������ȥ��
�÷�Ӧ�����ӷ���ʽΪCr
3++3OH
-=Cr��OH��
3����Ư�۵���Ч�ɷ���Ca��ClO��
2��
�ʴ�Ϊ��������Cr
3++3OH
-=Cr��OH��
3����Ca��ClO��
2��
��3����ʳ����һ�����Ӻ���Ԫ�ص����ʡ���пԪ�ص����ʣ���ʳ���в���Ҫ�Ӻ���Ԫ�ص����ʣ��ʴ�Ϊ��C��
�ڵ��ۡ���ά�ض����ڶ��ǣ��ʴ�Ϊ��A��
�۸�ð����ʱӦ�������ס�ֹ�ȵ�ҩ��簢˾ƥ�֣��ʴ�Ϊ��B��
��4���ٹ�����ˮ�����ͨ�������õ���ԭ��Ϊʯ��ʯ���ʴ�Ϊ��ʯ��ʯ��
�ھ۱�ϩ�ĵ���Ϊ��ϩ�������ϩ�Ľṹ��ʽ��

���ʴ�Ϊ��

��
�۸����ĸ�ʴ��Ҫ����������ʴ�������������õ��ӣ��缫��ӦΪO
2+4e
-+2H
2O=4OH
-�����ڴ����ϼӱ������õĽ���������������������Zn��Fe��Cu����Ӧѡп�飬
�ʴ�Ϊ��O
2+4e
-+2H
2O=4OH
-��п�飮
��������1���ٶ�����̼���������ڿ�����Ⱦ�
�ڿ�������Դ�����Ͽɸ��ƻ���������
��2�����ݷ�Ӧ��������������д��ѧ��Ӧ����ʽ��
��3����ʳ����һ�����Ӻ���Ԫ�ص����ʡ���пԪ�ص����ʣ�
�ڵ��ۡ���ά�ض����ڶ��ǣ�
�۸�ð����ʱӦ�������ס�ֹ�ȵ�ҩ��簢˾ƥ�֣�
��4���ٹ�����ˮ�����ͨ�������õ���ԭ��Ϊʯ��ʯ��
�ھ۱�ϩ�ĵ���Ϊ��ϩ��
�ۢ۸����ĸ�ʴ��Ҫ����������ʴ�������������õ��ӣ����ڴ����ϼӱ������õĽ�����������
���������⿼��֪ʶ��϶࣬ע�ػ�����Ⱦ����ѧ���������ϵ����Ϥ�����Ļ�����Ⱦ�����ע�������������û�ѧ֪ʶ����������е����⼴�ɽ����Ŀ�ѶȲ���

 2CaSO4+2CO2��
2CaSO4+2CO2�� 2CaSO4+2CO2��
2CaSO4+2CO2��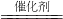 N2+2CO2��
N2+2CO2��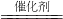 N2+2CO2��
N2+2CO2�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
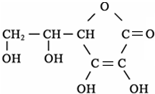 �������ʽΪ
�������ʽΪ ����־����
����־����