����Ϊѡ���⣬����A��B���⣮ѡѧ����ѧ�����ģ��Ŀ�����A�⣬ѡѧ���л���ѧ������ģ��Ŀ�����B�⣬ÿλ����ֻ��ѡ��1�⣮�����ⶼ��������A��Ʒ֣�
A������ѧ�����
��1��ʳƷ��ҩƷ��ϵ�˵�����ͽ�����
���г������۵�ʳ��Ʒ�ֺ࣮ܶ����ʳ���У������ӵ�Ԫ�ز��������������Ԫ�ص���
C
C
������ĸ����
A����п�� B���ӵ��� C���Ӹ���
����ά�ر���Ϊ������Ӫ���ء���ʳ���е���ά����Ȼ����Ϊ�����ṩ���������ܴٽ������䶯�������ų��к����ʣ��ӻ�ѧ�ɷֿ�����ά����һ��
A
A
������ĸ����
A������ B�������� C��֬��
��ijͬѧ��ð���գ����ɷ�����������ҩƷ��������
B
B
������ĸ����
A����Ƽ �� B����˾ƥ�� ��C������ҩ
��2���������������Ļ�����û�е����ʾ�û����������ش��������⣺
����֬�������������ø��������ˮ��Ϊ��֬�����
����
����
��д���ƣ����������������ɶ�����̼��ˮ���ṩ����������Ϊ�ϳ����������������ʵ�ԭ�ϣ�
����������ĵ�������θ����ø���ȵ���ø�������£�ˮ���
������
������
�����������պ����½�ϳ���������ĵ����ʣ������ڵ�����Ҳ�ڲ��Ϸֽ⣬������������ų����⣮��������һ���������ܷ������ԣ��Ӷ�ʧȥ�������ԣ���һ�������ͭ��Һ����ʱӦ�����������Ĵ�����
����ţ�̡�������ʵȸ��������ʵ�ʳƷ
����ţ�̡�������ʵȸ��������ʵ�ʳƷ
��
��ά����C��һ����Ҫά���أ��ܷ��λ�Ѫ�����õ�����Һ����ˮΪ�Լ�����֤ά����C���л�ԭ�Ե�ʵ�������������
ȡ����������Һ���������ε�ˮ����ɫ���ټ���ά����C������Һ��ɫ��ȥ
ȡ����������Һ���������ε�ˮ����ɫ���ټ���ά����C������Һ��ɫ��ȥ
��
��3��������������������ͷ�չ����Ҫ���ʻ�����
����ͨ�����������г��õĹ����β��ϣ�����Ҫ�ɷ���Na
2SiO
3��CaSiO
3��
SiO2
SiO2
���ѧʽ����������ͨ����ʱ��ʯӢ��̼���Ƹ����·�Ӧ�Ļ�ѧ����ʽΪ
��
��ͨ��ʹ�õĸ���������
̼
̼
�ĺϽ�Ϊ��ǿ�������Ŀ���ʴ����������һ�֡��������ķ���������������ʹ����������������һ�����ܵı�Ĥ����ɷ�Ϊ
Fe3O4
Fe3O4
���ѧʽ��������п���г���Ȧ�Ʋ㱻����ʱ������ʴ�ٶȱ�п
��
��
����족����������
����Ȼ�������������߷��ӣ���߷���������˫�����ϻ�����ҵ�ϳ������뺬����������ת���Ϊ���͵���״���ӵ�
��
��
��
B�����л���ѧ��
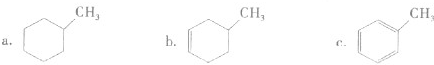
��1���л��ﺬ�еĹ����Ų�ͬ������Ҳ�в��죮
��1mol�����л������2mol�嵥�ʷ����ӳɷ�Ӧ����
B
B
������ĸ����
A����ϩ B����Ȳ C������
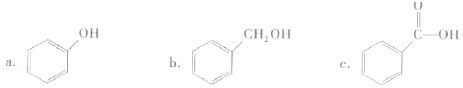
�������л����У��˴Ź�������ֻ��һ�����շ����
A
A
������ĸ����
A���� B������ C��������
�������л����У��ܷ���������Ӧ����
C
C
������ĸ����
A���������� B���Ҵ� C��������
��2������ʽΪC
4H
10O�����ڴ���ͬ���칹����
4
4
�֣�����һ�ֲ���������ȩ��ͪ�����Ľṹ��ʽ��
����һ��û��֧�����ܱ�������ȩ��д���������ᷢ��������Ӧ�ķ���ʽ
��
��3����ѧ�ҳ����ý�ҩ�������ڸ߷��������ϣ��Ƴɻ��ͳ�Чҩ���֪ij�ֽ�����ʹ��ҩ���ṹ��ʽ��ͼ�ף��������ӵ��߷��Ӿۺ���B�ϣ��γɻ��ͳ�Чҩ��C��ͼ�ң�
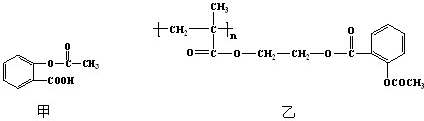
�ٷ��Ӿۺ���B�Ľṹ��ʽΪ
��
��A��B��Ӧ����C���л���Ӧ������
������Ӧ
������Ӧ
��
��A��ˮ���
��
CH3COOH
CH3COOH
��д�ṹ��ʽ����



 С�����ϵ�д�
С�����ϵ�д�









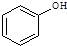 ��
��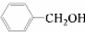 ��Ӧѡ��
��Ӧѡ��

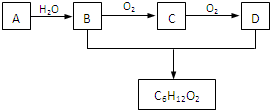


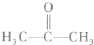
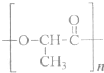


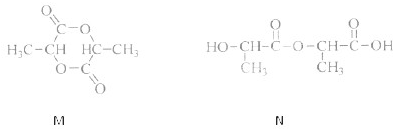
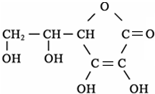 �������ʽΪ
�������ʽΪ