
����Ŀ������˵����ȷ���� ( )
A. CH4(g)+2O2(g)��CO2(g)+2H2O(g)��H=-801.3kJmol-1 ���ۣ�CH4��ȼ����Ϊ801��3kJ/mol
B. ϡ��Һ����H+(aq)+OH-(aq)=H2O(l)��H=-57.3kJ/mol ���ۣ��������백ˮ��ϡ��Һ��Ϻ�������1mol H2O�����ų�57��3kJ������
C. Sn(s����)![]() Sn(s����)��H��+2.1kJ/mol (����Ϊ��ĩ״) ���ۣ�����Ʒ�ں���Ķ�������ת��Ϊ��������
Sn(s����)��H��+2.1kJ/mol (����Ϊ��ĩ״) ���ۣ�����Ʒ�ں���Ķ�������ת��Ϊ��������
D. C(s��ʯī)+O2(g)=CO2(g) ��C(s�����ʯ)+O2(g)=CO2(g) ��H����395kJ/mol ���ۣ���ͬ�����½��ʯ���ʱ�ʯī�ȶ�
���𰸡�C
��������
A��ȼ�����������ȶ��IJ���(Һ̬ˮ)�ų�����������������̬ˮ����A����B����ˮ��������ʣ��������������������Էų�������С��57.3 kJ������ϡ�����백ˮ��Ӧ���к��ȴ���-57.3 kJ/mol����B����C����Sn(s����)![]() Sn(s����)��֪���¶ȵ���13.2��Cʱ������ת��Ϊ�����������Է�ĩ״���ڣ���C��ȷ��D���ɢ�C(s��ʯī)+O2(g)=CO2(g)��H1=-393.5kJmol-1����C(s�����ʯ)+O2(g)=CO2(g)��H2=-393.0kJmol-1�����-�ڵã�C(S��ʯī)=C(S�����ʯ)��H=+1.9kJmol-1�����ʯ��������ʯī�������������ʵ�������Խ��Խ���ȶ�����ʯī�Ƚ��ʯ�ȶ�����D����ѡC��
Sn(s����)��֪���¶ȵ���13.2��Cʱ������ת��Ϊ�����������Է�ĩ״���ڣ���C��ȷ��D���ɢ�C(s��ʯī)+O2(g)=CO2(g)��H1=-393.5kJmol-1����C(s�����ʯ)+O2(g)=CO2(g)��H2=-393.0kJmol-1�����-�ڵã�C(S��ʯī)=C(S�����ʯ)��H=+1.9kJmol-1�����ʯ��������ʯī�������������ʵ�������Խ��Խ���ȶ�����ʯī�Ƚ��ʯ�ȶ�����D����ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ������£�NCl3��һ����״Һ�壬����ӿռ乹����NH3���ƣ����ж�NCl3��NH3���й�������ȷ����( )
A. ������N��Cl��������CCl4������C��Cl���������
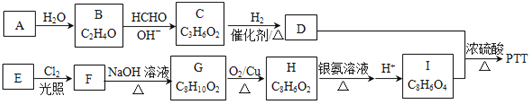
B. �ڰ�ˮ�У���NH3��H2O�����(�á�������ʾ)����γ�NH3��H2O���ӣ���NH3��H2O�ĽṹʽΪ��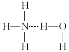
C. NCl3�����ǷǼ��Է���
D. NBr3��NCl3�ӷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯ��ʯ���Կ���CaO��FeO��Fe2O3��Al2O3��SiO2��ɡ���ҵ�϶�������ۺ����õ��������£�

��1���÷���ʽ��ʾʢ������������Һ���Լ�ƿ�����ò�������ԭ��________��
��2����Һ���г���Ca2+�⣬�����ܺ��еĽ�����������_______________��
��3���������NaOH�μӷ�Ӧ�����ӷ���ʽ��________________�������������ֽ����Ŀ����_____________________��
��4���ڹ�ҵ�����У������ͨ�����CO2�������������ԭ����___________��д�������ͨ�����CO2�����ӷ���ʽ _________________________________��
��5�������ʵ��֤����ʯ���к���FeO���Լ���ѡ��˵��ʵ�����������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���۶Ա��������������PTT����һ��������������;�����ά���Ϻ������Ծ������ϣ��ǵ�ǰ���������¿��������Ÿ߷����²��ϡ�PTT��һ�ֺϳ�·����ͼ��ʾ��
��֪��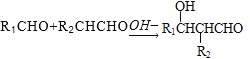
�Իش��������⣺
��1����֪A��B�Ǽӳɷ�Ӧ��A�ķ���ʽΪ________��C�к��еĹ�������_________�������ƣ���
��2���л���D������Ϊ_______________________������ϵͳ������������
��3��������E��һ�ȴ���ֻ��2�֣���E�Ľṹ��ʽ______________��
��4����D��I�ϳ�PTT�Ļ�ѧ����ʽΪ______________���÷�Ӧ�ķ�Ӧ����Ϊ____________��
��5���л���G��ͬ���칹���кܶ��֣�ͬʱ��������������ͬ���칹����________�֡�
������FeCl3��Һ������ɫ��Ӧ �ڱ�����ֻ������ȡ���� �۷����к���������OH��
���к˴Ź���������ʾ��5��壬�ҷ����֮��Ϊ3��2�� 2�� 2�� 1����__________����ṹ��ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��500mLNaNO3��Cu(NO3)2�Ļ����Һ��c(Na��)Ϊ0.2 mol��L��1����Pt���缫������Һ����ͨ��һ��ʱ����������ռ���2.24 L����(��״����)���ٶ�������Һ�����Ϊ500 mL������˵����ȷ����
A. ԭ�����Һ��c(NO3-)��0.4 mol��L��1
B. ����������������5min,��ͨ���ĵ���Ϊ0.2 ��NA��1.60��10��19C
C. ���õ���Cu������Ϊ12.8g
D. ������Һ��pH=1��Ҫ��ָ�ԭ״���ɼ�0.05mol��CuCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
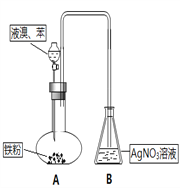
����Ŀ�������£�������������ʢ�ŵ�Һ����( )
A. ϡ���� B. CuSO4��Һ C. FeCl3��Һ D. NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ���绯ѧ���̵�ʾ��ͼ����ش��������⣺
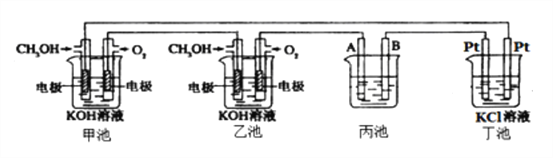
��1��ͨ��CH3OH�ĵ缫�ĵ缫��ӦʽΪ__________________��
��2��������ͭ�ľ����أ����һ��ʱ����ձ��е���ҺŨ�Ȼ�______����������С������������A�缫��Ӧʽ_______________________����֪��ͭ�к���Zn��Ag���ʣ���
��3�������е��з�̪��ʵ�鿪ʼ��۲쵽������_____________________�����ز�����Ĥ���,�������������ܻ���KOH��Һ�Ӵ����õ�KClO��H2������Ӧ�Ļ�ѧ��Ӧ����ʽΪ____________________________________________________��
��4�����ס��������������ͨ������Ϊ20L����״�������ҷ�Ӧ��ȫ����������ͨ�����صĵ���Ϊ__________�������ڳ���F=9.65��l04Cmol-1������������ܲ�������������Ϊ______L����״������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ���㷺����ʳƷ��ζ������ij�ֺϳ�·��������ʾ��
���㷺����ʳƷ��ζ������ij�ֺϳ�·��������ʾ��
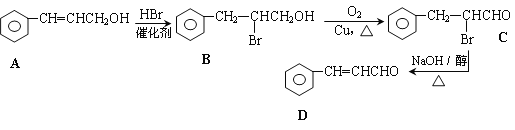
�ش��������⣺
��1��B�еĹ���������Ϊ_______________
��2��C����D�ķ�Ӧ����Ϊ_____________
��3��������·���У�HBr��������__________________________________________________
��4�� ![]() ���Ӽ�ȩ����ȩ����ȩ��ѡ��ԭ�ϣ���ԭ����ѡ�������������ϳ�·�ߣ�д���������ϳ�C(CH2OH)4�ĺϳ�·��_____________________________________________________________
���Ӽ�ȩ����ȩ����ȩ��ѡ��ԭ�ϣ���ԭ����ѡ�������������ϳ�·�ߣ�д���������ϳ�C(CH2OH)4�ĺϳ�·��_____________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ�������N������ʹ������ҩ�����ã���ϳ�·�����£�

��1��A��ϵͳ����Ϊ____________��E�й����ŵ�����Ϊ____________��
��2��A��B�ķ�Ӧ����Ϊ____________���ӷ�Ӧ����Һ̬�л���������ᴿB�ij��÷���Ϊ____________��
��3��C��D�Ļ�ѧ����ʽΪ________________________��
��4��C��ͬ���칹��W�������������칹���ɷ���������Ӧ����1 mol W�����2 mol NaOH������Ӧ������֮һ�ɱ������ɶ�Ԫȩ����������������W��____________�֣���W�ĺ˴Ź�������������壬����ṹ��ʽΪ____________��
��5��F��G�Ĺ�ϵΪ������ţ�____________��
a��̼���칹 b���������칹 c��˳���칹 d��λ���칹
��6��M�Ľṹ��ʽΪ____________��
��7�����������ϳ�·�ߣ���![]() Ϊԭ�ϣ��������·����Ʊ�ҽҩ�м���
Ϊԭ�ϣ��������·����Ʊ�ҽҩ�м���![]() ��
��

��·�����Լ�������1Ϊ____________��X�Ľṹ��ʽΪ____________��
�Լ�������2Ϊ____________��Y�Ľṹ��ʽΪ____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com