
��Ȼ��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl����SO42����Br����CO32����HCO3�������ӡ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤����������ͼ��ʾ������˵��������ǣ� ��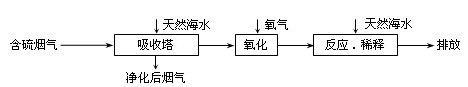
A����Ȼ��ˮpH �� 8��ԭ�������ں�ˮ�е�CO32����HCO3��ˮ�� |
B����������������������H2SO3��HSO3����SO32������������SO42�� |
C������Ӧ��ϡ�͡�ʱ����Ȼ��ˮ��Ŀ�����кͣ�ϡ�;�������ˮ�����ɵ��� |
D�����ŷš������ĺ�ˮ��SO42�������ʵ���Ũ�����������������Ȼ��ˮ��ͬ |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����˵��������ǣ�������
����˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
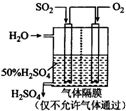 ���ᱻ��Ϊ����ҵ֮ĸ����������ڹ�ҵ�����е���Ҫ��λ�����ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ��������Ĺؼ����裮һ���¶��£���һ�������������Ϊ2L���ܱ������г���2.0mol SO2��g����1.0mol O2��g����������Ӧ��SO2��g��+
���ᱻ��Ϊ����ҵ֮ĸ����������ڹ�ҵ�����е���Ҫ��λ�����ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ��������Ĺؼ����裮һ���¶��£���һ�������������Ϊ2L���ܱ������г���2.0mol SO2��g����1.0mol O2��g����������Ӧ��SO2��g��+| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl����SO42����Br����CO32����HCO3�������ӡ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤����������ͼ��ʾ������˵��������ǣ� ��
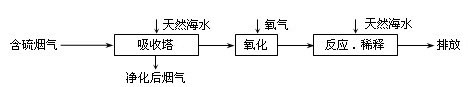
A����Ȼ��ˮpH �� 8��ԭ�������ں�ˮ�е�CO32����HCO3��ˮ��![]()
B����������������������H2SO3��HSO3����SO32������������SO42��![]()
C������Ӧ��ϡ�͡�ʱ����Ȼ��ˮ��Ŀ�����кͣ�ϡ�;�������ˮ�����ɵ���![]()
D�����ŷš������ĺ�ˮ��SO42�������ʵ���Ũ�����������������Ȼ��ˮ��ͬ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȼ��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl����SO42����Br����CO32����HCO3�������ӡ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤����������ͼ��ʾ������˵��������ǣ� ��
A����Ȼ��ˮpH �� 8��ԭ�������ں�ˮ�е�CO32����HCO3��ˮ��
B����������������������H2SO3��HSO3����SO32������������SO42��
C������Ӧ��ϡ�͡�ʱ����Ȼ��ˮ��Ŀ�����кͣ�ϡ�;�������ˮ�����ɵ���
D�����ŷš������ĺ�ˮ��SO42�������ʵ���Ũ�����������������Ȼ��ˮ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ��У2009-2010ѧ��ȸ�����һ����������ѧ������ ���ͣ�ѡ����
��Ȼ��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl����SO42����Br����CO32����HCO3�������ӡ���������ȼú�ŷŵĺ�SO2�����������ú�ˮ�����乤����������ͼ��ʾ������˵��������ǣ� ��
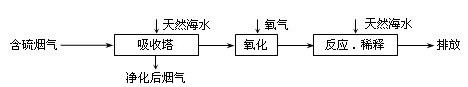
A����Ȼ��ˮpH �� 8��ԭ�������ں�ˮ�е�CO32����HCO3��ˮ��
B����������������������H2SO3��HSO3����SO32������������SO42��
C������Ӧ��ϡ�͡�ʱ����Ȼ��ˮ��Ŀ�����кͣ�ϡ�;�������ˮ�����ɵ���
D�����ŷš������ĺ�ˮ��SO42�������ʵ���Ũ�����������������Ȼ��ˮ��ͬ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com