
������������Ÿ��ָ�������;���������﹤��ѧ����������ӿ�ʯ����ȡ������ͭ��һ�ֺ��м�ֵ�Ľ��������Դ�ͭ������ȡ������Dz���ijЩϸ�����ÿ����е�����������ͭ��ʯ���Ѳ����Ե���ͭת���ɿ��ܵ�����ͭ������ϸ����ȡͭ���������̣�������ϸ�����ڵ���ʯ����ʯ���У�������ˮ�Դٽ�ϸ�������������ǵ����������У��������ɵ�����ͭ�γɵ�Ũ�ȵ���Һ������ʯ�ѵĵײ����ٴ�������Һ����ȡ����ͭ��ˮѭ��ʹ�ã��ٻص���ʯ���С���������10%��ͭ�������ַ��������ġ�
�Իش��������⣺
��1��ϸ������ͭ����Ϊ����ͭ�Ĺ���������ʲô���ã� ��
��2��������ͭ��Һ����ȡͭ���������õķ����� ����Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����ͨ����ͭ�ķ������ڿ�����ȼ����ͭ����������һ����̬��������ԱȽ����ַ�������ȱ�㡣
��
��4����һ����������������ϡH2SO4��������CuO�Ƴɵ���ͭ����������������ַ�������Fe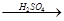 H2
H2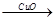 Cu����CuO
Cu����CuO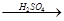 CuSO4
CuSO4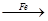 Cu
Cu
����ʵ��ԭ����в������������Ƶõ���ͭ�����ıȽ��У���ȷ���� (����)
| A���ٶ� | B���ڶ� | C����� | D�����ж� |
(1�������� ��2��������ͭ��Һ�����������ͭ�û�������Fe+CuSO4=FeSO4+Cu
��3����ͨ����������ͭ�������̳��Ͷ�������������Ⱦ���������﹤�̸����ŵ㣬�����ŵ��dz� ���ͣ�Ч��ã�������Ⱦ�̶�С��
��4��B (5)Cu2S��O2��ͭ ��6���Ƚ������������������������ӣ��ٵ���pH��3-4��
�������������
��1��ϸ������ͭ����Ϊ����ͭ�Ĺ����У���δ���뷴Ӧ��������ã�
��2�����÷��������û����ȼ���־��á�Fe+CuSO4=FeSO4+Cu��
��3����ͨ����������ͭ�������̳� ��SO2������Ⱦ���������﹤�̸����ŵ㣬�����ŵ��dzɱ��͡�Ч��ã�������Ⱦ�̶�С��
��4���������Ļ�ѧ����ʽΪ����CuO+H2SO4�TCuSO4+H2O��Fe+CuSO4�TCu+FeSO4��Fe+H2SO4 �TFeSO4+H2����H2+CuO  Cu+H2O����������������ԭ����ͭʵ�鿪ʼʱ��ѡͨ��һ�����������ų�װ���еĿ�����ʵ�����ʱ��Ҫͨһ����������ֹ���ɵ�ͭ������������ͬ���������������������������ͨ��ͣ��ͭ����ͬ�ģ��������ڲ����������˷ѣ����¶�Ӧ��ͭ���٣��ʢ����ɵ�ͭ�࣮
Cu+H2O����������������ԭ����ͭʵ�鿪ʼʱ��ѡͨ��һ�����������ų�װ���еĿ�����ʵ�����ʱ��Ҫͨһ����������ֹ���ɵ�ͭ������������ͬ���������������������������ͨ��ͣ��ͭ����ͬ�ģ��������ڲ����������˷ѣ����¶�Ӧ��ͭ���٣��ʢ����ɵ�ͭ�࣮
��5)��ҵ�Ͽ���Cu2S��O2��Ӧ��ȡ��ͭ��Ԫ�ػ��ϼ�ͭԪ�ػ��ϼ۽��ͣ���Ԫ�ػ��ϼ����ߣ���������Ԫ�ػ��ϼ۽��ͣ�˵����ͭ����ԭ����Ԫ�ر�������������������ԭ��ӦΪCu2S+O2�T2Cu+SO2���÷�Ӧ��������ΪO2��Cu2S����⾫��ͭ����ͭ����������ͭΪ�������������ҺΪ������ͭ�Σ�
��6��������Ŀ��Ϣ������Fe2+��Fe��OH��2��ʽ��ȫ������pH��9.6����ʱCu2+��Cu��OH��2����ʽ��ȫ����������Fe2+������Fe3+��Fe3+��Fe��OH��3��ʽ��ȫ������pH��3��4����ʱCu2+û���γɳ������ȳ�ȥ��ȥ�����ʣ�Ҳû�г�ȥԭ���ʣ�
���㣺���������ۺϴ��壬��Ϣ����֪ʶ��ࡣ��������Ŀʱ��Ҫ����С������һ���ơ�ÿ��С���漰��֪ʶ�㲻�ã������ò��õĻ�ѧ֪ʶ�ͻ�ѧԭ��ȥ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼʵ��װ��������֤ijЩ���ʵ����ʡ����Թ�A��װ�������Ĺ���NaHCO3��DΪ�̶������ӲֽƬ���Իش��������⣺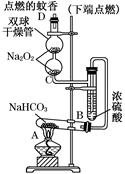
��1����A�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��___________________��
��2��Bװ�õ�������__________________________________��
��3����˫�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
��4��˫�������ڹ۲쵽��ʵ��������__________________________
____________________________________________________________________��
����ʵ������˵��________________________________________________________��
��5������������ڵ�Na2O2����Na2O����˫�������ڹ۲쵽��ʵ��������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�� Mg2����Al3����Һ10mL�������еμ�a mLC1mol��L��1NaOH֮�ijɵμ�C2mol��L��1HCl�����ó���Y��mol���������Լ������V��mL����Ĺ�ϵ����ͼ��ʾ���ݴˣ��ش��������⣺
��1��C��D�����е����ӷ���ʽ ��
��2��n(Mg2��)/ n(Al3��) = ��
��3��C1/C2= ��
��4��������NaOH��Һ�����a= mL��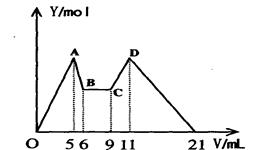
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɷ����Ʊ�FeCl2����Ҫ�������£�
����ͼ��ʾ�����Ʊ�FeCl3?6H2O 
��1�����в�������ĵ���ʽ�� ��
��2�������ӷ���ʽ��ʾ���̢���ϡ��������� ���ڸù�����Ҫ��������Һ�в������ᣬĿ���� ��
����FeCl3 ? 6H2O�Ƶø���FeCl2�Ĺ������£�
������ʢ��FeCl3 ? 6H2O�������м���SOCl2�����ȣ������ˮFeCl3
��������ˮFeCl3���ڷ�Ӧ���У�ͨ����в���������һ��ʱ�����ȣ�����FeCl2
�����ռ�FeCl2�����汸��
��3�� SOCl2��ˮ�Ӵ���Ѹ�ٲ���������SO2�����ȷֽ�FeCl3 ? 6H2O���ܵõ���ˮFeCl3�������袡�пɵõ���ˮFeCl3����ϱ�Ҫ��ѧ����ʽ���͵õ���ˮFeCl3��ԭ�� ��
��4�����̢��в���FeCl2�Ļ�ѧ����ʽ�� ��
��FeCl2�İ�װ�����а�ȫע����������������£�
| Ʒ �� | �Ȼ����� |
| �������� | ��ɫ���������ױ�ɻ�ɫ������ˮ���и�ʴ�ԡ��� |
| ע������ | ����Ӵ���������ȣ�����ϩ����Ͱ��װ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ��һ���������ϵ�dz����е���ɫ�����������£�Cu2+����Һ���ȶ���Cu+�����������������绯��Ӧ��2Cu+=Cu2++Cu�������+1��ͭ�Ļ�������������磺Cu20��Cul��CuCl��CuH �ȡ�
��1����CuCl2��Һ����μ������KI��Һ�����ܷ����ķ�Ӧ�У�
2Cu2++4I-=2CuI������ɫ��+I2; 2Cu2++4I-+2Cl-=CuCl������ɫ��+I2
��֪��������Ksp(CuCl)=1.20��10-6(mol/L)2; Ksp(CuI)=5.06��10-12(mol/L)2���ɴ��ƶ�������Ӧ������Ҫ������Ļ�ѧʽ��______��
��2����CuH�м������ϡHC1�����������ɣ��÷�Ӧ�����ӷ���ʽΪ______��
��3������ͭ����Cu2S��FeS�ۺϳɺ�Cu 18%��20%��һ�����ʣ������ۼ������{���´����������ͭ�е�Cu2S������ΪCu2O�����ɵ�Cu2O��Cu2S��Ӧ���ɴ�ͭ������������Ӧ�Ļ�ѧ����ʽ�ֱ���______��______��
��4�������£���0.20 mol ? L-1����ͭ��Һ�м�������������Һ������dz��ɫ������ͭ����������Һ��pH = 6ʱ��c(Cu2+)=______mol.L?1��[��֪��Ksp(CuI)=2.2��10-20(mol/L)3]
��5����0.80 gCuSO4 ? 5H2O��Ʒ���ȷֽ⣬����ˮ�����й����������¶ȵı仯����ͼ��ʾ��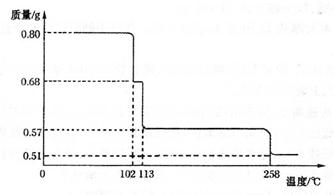
��ȷ��110��Cʱ�������ʵĻ�ѧʽ____________����Ҫ��д���ƶϹ��̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ľ���֮һ��
��1��Ũ���ᡢŨ���������������������ԭ���� ��
��2����������ҪӦ���ڻ���ƽ�������ҵ��������ˮ�Ȼ������⻯��ﮣ�LiAlH4�����л��ܼ��з�Ӧ�Ƶ�����������ѧ����ʽ���£�3LiAlH4+AlCl3="4Al" + 3LiCl + 6H2��
�÷�Ӧ��������Ϊ ��
��3���⻯���ƣ�NaAlH4����һ����Ҫ�Ĵ�����ϣ���֪��
NaAlH4(s)=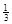 Na3AlH6 (s)+
Na3AlH6 (s)+ 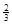 Al (s) + H2(g) ��H��+ 37 kJ��mol��1
Al (s) + H2(g) ��H��+ 37 kJ��mol��1
Na3AlH6(s)="3NaH(s)+" Al (s) + 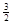 H2(g) ��H��+ 70.5 kJ��mol��1
H2(g) ��H��+ 70.5 kJ��mol��1
��NaAlH4(s)=" NaH(s)" + Al (s) +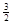 H2(g) ��H�� ��
H2(g) ��H�� ��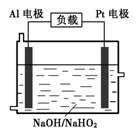
��4����֪H2O2��һ�����ᣬ��ǿ������Һ����Ҫ��HO2����ʽ ���ڡ�Ŀǰ�о��Ƚ����ŵ�Al��H2O2ȼ�ϵ�أ���ԭ������ͼ��ʾ������ܷ�Ӧ���£�
2Al+3HO2��+3H2O =2[Al(OH) 4]��+OH��
��������ӦʽΪ ��
������ͨп�̸ɵ����ȣ���������ͬ�����ĸ�����������ʱ��Al��H2O2ȼ�ϵ�ص����۷ŵ���ԼΪ��ͨп�̸ɵ�ص�______����
��Al�缫�ױ�NaOH��Һ��ѧ��ʴ�����Ǹõ��Ŀǰδ���ƹ�ʹ�õ�ԭ��֮һ����Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣�����A�����ڹ�ҵ���������ᣬ�����ʼ�ת������ͼ������CΪ����ɫ��
ĩ��DΪ��ʹƷ����Һ��ɫ����ɫ���塣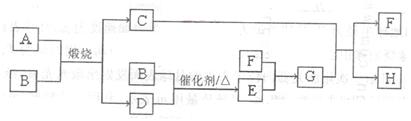
��ش�
��1��F�ĵ���ʽΪ_________��
��2�����������C��D��������Ϊ5:8����A�Ļ�ѧʽΪ______________��
��3����Dͨ��H��Һ�з�����Ӧ�����ӷ���ʽΪ_____________��
��4�����ʵ���Ũ�Ⱦ�Ϊ0.lmol/L��G��H�Ļ����Һ�У�����Ũ���ɴ�С��˳��
Ϊ____________��
��5�������õ��ij������Һ�ķ���ͬʱ�Ʊ�G��Ũ��Һ��Ũ����������Һ����ͼ��
ʾ����
��a��Ϊ��Դ��_________�������������������
��N���ռ�������Ϊ__________���ѧʽ����ͬ������
Q���ռ���Ϊ_________Ũ��Һ��
��ij���ʵı�����Һ���ѡ��___________�����Լ����ƣ���
�������ĵ缫��ӦʽΪ____________��
��6������H�н��������ӵķ���___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
ʵ���Ҳ���MgCl2��AlCl3�Ļ����Һ�������ˮ��Ӧ�Ʊ�MgAl2O4����Ҫ��������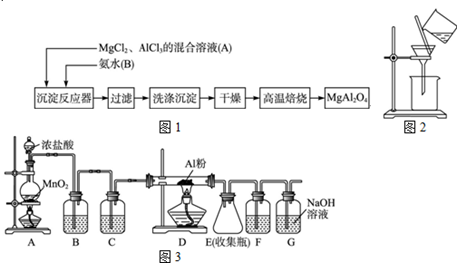
��1��ΪʹMg2+��Al3+ͬʱ���ɳ�����Ӧ���������Ӧ���м��� ���A����B�����ٵμ���һ��Ӧ��Ʊ�MgAl2O4�����У����±���ʱ������Ӧ�Ļ�ѧ����ʽ ��
��2������ͼ��ʾ�����˲����е�һ�������� ��
��3���ж������г����Ƿ�ϴ�����õ��Լ��� �����±���ʱ������ʢ�Ź�������������� ��
��4����ˮAlCl3��183������������ʪ��������������������ʵ���ҿ�������װ���Ʊ���װ��B��ʢ�ű���NaCl��Һ����װ�õ���Ҫ������ ��F���Լ��������� ����һ������װ���ʵ��Լ���Ҳ����F��G�����ã���װ����Լ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
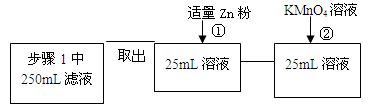
��16�֣�ij�о�С��Ϊ��̽��������ˮ�������ķ�Ӧ�IJ������������ʵ�飺����ͼ��ʾʵ��װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��ʯ�������²���ˮ������Ӧ����
��1����μ���װ�õ������ԣ�
��2��д������ˮ������Ӧ�Ļ�ѧ����ʽ
��3����֤�����������Ԫ�صļ�̬
��ѡʵ���������Լ����ձ����Թܡ���������ҩ�ס��ιܡ��ƾ��ơ��ԹܼУ�1mol/L CuSO4��3mol/L H2SO4��3mol/L HNO3��30%H2O2��0.01mol/L KMnO4��20%KSCN������ˮ��
�ڴ���ϰ��±��ĸ�ʽд��ʵ�鲽�衢Ԥ����������ۡ�
| | ʵ�鲽�� | Ԥ����������� |
| ����1 | ȡ��Ӧ�����Ĺ���ag���Թ��У�����������1mol/L CuSO4��Һ�����������Һ���롢ϴ�Ӻ���������еμ������� �����ܽ⣬���˺���Һ���250mL��Һ�����á� | |
| ����2 | ȡ��������1����Һ���Թ��У� | |
| ����3 | ȡ��������1����Һ���Թ��У� | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com