
Ԫ�����ڱ��������о��������ʵ���Ҫ���ߣ��±���Ԫ�����ڱ���һ���֣����û�ѧ����ش��������⣺
(1)�١��ߺ�Ԫ��ԭ�Ӱ뾶������____������������Ӧ��ˮ������������ǿ����________________.
(2)д���ɢ�-�ߺ�Ԫ���еļ�������Լ��Ե��εĻ�ѧʽ____________________����д��2�ּ��ɣ����������ӷ���ʽ��ʾ����һ���Լ��Ե�ԭ��____________________.
(3)д���ۺ�Ԫ�ص�������������-�ߺ�Ԫ���н�������ǿ��Ԫ�ص�����������ˮ���ﷴӦ�����ӷ���ʽ_____________________��
(4) As��Ԫ�����ڱ��е�λ����________________��
��As��ԭ�ӽṹʾ��ͼΪ________�����⻯��ĵ���ʽΪ___________.
(6)Y���ɢڢޢ�����Ԫ����ɣ�����ˮ��Һ��һ�������г�������������As������Y��ˮ��Һ��Ӧ����������ۺ�����(H3AsO4 )��д���÷�Ӧ�����ӷ���ʽ_____________________,
������1mol��ԭ��ʱת�Ƶ��ӵ����ʵ���Ϊ______________________��
��Na��HClO4��
��Na2CO3��NaHCO3��Na2C2O4��HCOONa��NaAlO2��CH3COONa��CO32����H2O HCO3����OH��
HCO3����OH��
��Al2O3��2OH����2AlO2����H2O
�ȵ������ڵڢ�A��
��As��ԭ�ӽṹʾ��ͼ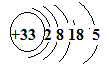
AsH3�ĵ���ʽ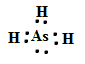
��2As��5ClO����3H2O��2H3AsO4��5Cl��
��������
�����������Ԫ�����ڱ��У�ͬһ�壬���ϵ��£��뾶Խ��Խ��ͬһ���ڣ������ң��뾶Խ��ԽС�������ڢ١��ߺ�Ԫ��ԭ�Ӱ뾶������λ�����·���Na��ͬһ���ڣ�����������Ӧ��ˮ��������Դ�����������ǿ���Ҹ�������Ŀǰ��֪����������������ǿ���ᡣ
����ǿ����������γɵ��ζ��Լ��ԡ���Ҫ���������������ӵ�ˮ�⡣
�Ǣۺ�Ԫ���������������������������������-�ߺ�Ԫ���н�������ǿ��Ԫ����λ�����ڱ������·����ƣ�ʵ���Ͼ���д���������������Ƶķ�Ӧ���ӷ���ʽ��
��Asλ�����ڱ��еĵ������ڵڢ�A�塣
��As��ԭ�ӽṹʾ��ͼ�ǣ�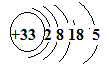 �����⻯��ĵ���ʽΪ��
�����⻯��ĵ���ʽΪ��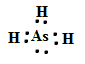
���ɢڢޢ�����Ԫ����ɵ��Ǵ������ƣ�
���㣺���ʽṹ��Ԫ�������ɡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
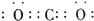
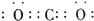
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | |||||||||||||||||
| �� | �� | �� | |||||||||||||||
| �� | �� | �� | |||||||||||||||
| Fe | As | ||||||||||||||||



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
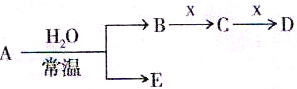
��ͼ��Ԫ�����ڱ���һ���֣�A��B��C��D��E��X�����ڱ�����Ԫ����ɵij������ʻ��
| �� |
|
|
|
|
|
|
|
| |||||||||
|
|
|
| �� | �� | �� |
|
| ||||||||||
| �� |
|
|
|
|
|
| �� |
| |||||||||
|
|
|
|
|
|
|
| Fe |
|
|
|
|
|
| As |
|
|
|
I��Ԫ�����ڱ��������о��������ʵ���Ҫ����
Y�ɢڢޢ�����Ԫ����ɣ�����ˮ��Һ�������г�������������As����Y��ˮ��Һ��Ӧ��������As����ۺ����ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ ��������1mol��ԭ��ʱ������ת���� ________mol��
����֪A��B��C��D��E��X������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ��
��1����EΪ�������A�Ļ�ѧʽΪ ��A��ˮ��Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
�� ��X�Ǽ�������Һ��C��������22������ʱ����C�ĽṹʽΪ ����ʾX�ʼ��Ե����ӷ���ʽΪ ��
�� ��XΪ��������ʱ����X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ
��2����EΪ�������壬DΪ��ɫ������A�Ļ�ѧʽ������ ��B���еĻ�ѧ����Ϊ ��C��X��Ӧ�����ӷ���ʽΪ ��
��3����BΪ�������壬D����ˮ������һ�������·������淴Ӧ������C��һ�ֿ�ȼ�����嵥�ʣ���ÿ��淴Ӧ�Ļ�ѧ����ʽΪ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ����ƽ�⣬���¶��·�Ӧ��ƽ�ⳣ��K=1��D��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ȱ������и�����һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
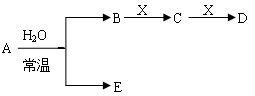
��ͼ��Ԫ�����ڱ���һ���֣�A��B��C��D��E��X�����ڱ�����Ԫ����ɵij������ʻ��
| �� | | | | | | | | | |||||||||
| | | | �� | �� | �� | | | ||||||||||
| �� | | | | | | | �� | | |||||||||
| | | | | | | | Fe | | | | | | | As | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ȱ������и�����һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��ͼ��Ԫ�����ڱ���һ���֣�A��B��C��D��E��X�����ڱ�����Ԫ����ɵij������ʻ��
|
�� |
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
�� |
�� |
�� |
|
|
||||||||||
|
�� |
|
|
|
|
|
|
�� |
|
|||||||||
|
|
|
|
|
|
|
|
Fe |
|
|
|
|
|
|
As |
|
|
|
I��Ԫ�����ڱ��������о��������ʵ���Ҫ����
Y�ɢڢޢ�����Ԫ����ɣ�����ˮ��Һ�������г�������������As����Y��ˮ��Һ��Ӧ��������As����ۺ����ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ ��������1mol��ԭ��ʱ������ת���� ________mol��
����֪A��B��C��D��E��X������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ��
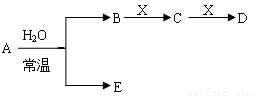
��1����EΪ�������A�Ļ�ѧʽΪ ��A��ˮ��Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
�� ��X�Ǽ�������Һ��C��������22������ʱ����C�ĽṹʽΪ ����ʾX�ʼ��Ե����ӷ���ʽΪ ��
�� ��XΪ��������ʱ����X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ
��2����EΪ�������壬DΪ��ɫ������A�Ļ�ѧʽ������ ��B���еĻ�ѧ����Ϊ ��C��X��Ӧ�����ӷ���ʽΪ ��
��3����BΪ�������壬D����ˮ������һ�������·������淴Ӧ������C��һ�ֿ�ȼ�����嵥�ʣ���ÿ��淴Ӧ�Ļ�ѧ����ʽΪ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ����ƽ�⣬���¶��·�Ӧ��ƽ�ⳣ��K=1��D��ת����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com