
���ҵĻ������÷����ܶ࣬���ú���Al2O3��SiO2������FeO xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3 18H2O�������������£�
18H2O�������������£�
��ش��������⣺
��1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��______________��
��2�������м��˵�KMnO4Ҳ����H2O2���棬����H2O2������Ӧ�Ļ�ѧ����ʽΪ_______________��
��3����֪��Ũ�Ⱦ�ΪO.1mol/L�Ľ��������ӣ������������������pH���±���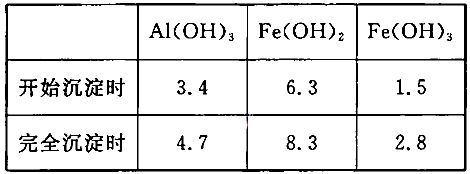
����۵�Ŀ����__________________________________________________________�����ڸ�Ũ���³�ȥ���Ļ��������pH�����Χ��___________��
��4����֪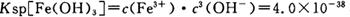 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��5�������ܷ�����Ӧ�����ӷ���ʽΪ__________________________________________��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_________��_________��
��6�������ݡ�һϵ�в���"�����������в����õ���___________������ţ���
| A�������� | B������ | C�������� | D���ƾ���E��©�� |
��1��6H+ + Al2O3=2Al3+ + 3H2O ��2��H2O2 + 2FeSO4 + H2SO4 =" Fe2(SO4)3" + 2H2O ����3���������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��2.8��3.4����4��4*10-2mol/L����5��3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ũ�������������Һ����6�� B
���������������1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��6H+ + Al2O3=2Al3+ + 3H2O ����2�������м��˵�KMnO4Ҳ����H2O2���棬H2O2��ǿ�����Ѷ�������������Ϊ���������ӣ�������Ӧ�Ļ�ѧ����ʽΪH2O2 + 2FeSO4 + H2SO4 =" Fe2(SO4)3" + 2H2O ����3����������ͼ��֪�ǽ������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��ȷ�������Ӳ�Ҫ�����������ʵ���pH��ΧΪ��2.8��3.4����4����֪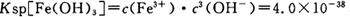 ��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������
��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������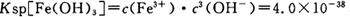 ��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ���ܷ�����Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6�������ݡ�һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ���ܷ�����Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6�������ݡ�һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
���㣺���⿼�����ӷ���ʽ����д��ʵ��ԭ���ķ����ͳ����ܽ�ƽ����ؼ��㡣


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ڹ�ҵ�Ͼ��й㷺��Ӧ�á�
I���ݱ�����һ������������FeO2����鷴Ӧ����ȡ���������Ľ��������䷴Ӧ���£�
Fe2O3(s)��3CH4(g)��2Fe(s)��3CO(g)��6H2(g) ��H��0
��1������Ӧ��2L���ܱ������н��У�5min��ﵽƽ�⣬��÷�Ӧ����Fe������Ϊl.12g����ö�ʱ����CH4��ƽ����Ӧ����Ϊ ________________��
��2����Ӧ�ﵽƽ����������������䣬���ı�ijһ���Ԫ�أ�����˵����ȷ����___(ѡ�����)��
a�������������v������ƽ�������ƶ�
b�����¶����ߣ�ƽ�ⳣ��K��С
c��������Fe2O3������ƽ�������ƶ�
d�����ӷ�Ӧ��ϵ�����߲���CO�������CH4��ת����
��ҵ����������������(��Ҫ�ɷ�ΪFe2O3��A12O3��SiO2��)Ϊԭ����ȡFe2O3������
�������£��Իش��������⣺
��3������i������A12O3�ܽ�����ӷ���ʽΪ________________��
��4������i����������Ҫ���еIJ���������__________________��
��5�������ͼ���жϲ��袢�е�����Һ��pH������_________________��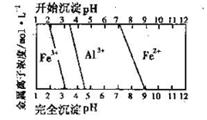
��6����֪
���㷴ӦFe2��(aq)��2HCO3��(aq) FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
��7���ڿ���������FeCO3�����������Ļ�ѧ����ʽΪ____________��
��8�������ѧ��ѧ֪ʶ�����������ʵ���������������������������ȡ����������һ�ּ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС����������(��Ҫ�ɷ�ΪA12 O3,������SiO2������������)��ȡ��������ұ������ԭ��,��ȡ�IJ�����������: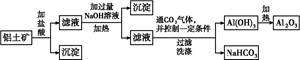
(1)�ڹ��˲�����,���ձ�����������,�����õ��IJ��������� ;ϴ�ӳ����IJ����� ��
(2)ʵ�����Ʊ����������ķ����ж��֡����ṩ��м������������Һ��ϡ��������ҩƷ,���Ʊ���������������,�����ҩƷ�������ٵĽǶȳ���,��Ƴ����ʵ�鷽��(�������ظ���),д���˷����з�����Ӧ�����ӷ���ʽ: ,�˷���������ҩƷ�����ʵ���֮����:n(Al)��n(H2SO4)��n(NaOH) = ��
(3)��ȤС����������������Ԫ�صļ�̬����̽��:ȡ��������,�������ϡ����,�����ܽ�;ȡ������Һ�μ�KSCN��Һ����ֺ�ɫ���ɴ˵ó���Ԫ�صļ�̬Ϊ+3�Ľ��ۡ���ָ���ý����Ƿ������˵������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������㷺�����л��ϳɡ�ӡȾ��ҵ�ȡ���ҵ��������Ϊԭ�ϣ���Ҫ�ɷ�ΪAl��������Al2O3��Fe2O3��SiO2��CaO��MgO�ȣ��Ʊ��������Ĺ����������£�
��֪��Al��OH��3�������ܽ��pH���±���
| Al��OH��3 | ��ʼ���� | ������ȫ | ������ʼ�ܽ� | �����ܽ���ȫ |
| pH | 3.3 | 5.0 | 7.8 | 12.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú�������������ͭ��ȡ�Ȼ�ͭ����(CuCl2��xH2O)�������²���:
��֪:��pHΪ4��5ʱ,Fe3+������ȫˮ�������,����ʱCu2+ȴ������ˮ�⡣
(1)������A��ѡ����������(����,��ͬ)��
��Cl2 ��H2O2 ��HNO3 ��KMnO4
(2)Ҫ�õ��ϴ��IJ�Ʒ,�Լ�B��ѡ������������
��NaOH ��FeO ��CuO ��Cu2(OH)2CO3
(3)����Һ�����ᾧ�õ��Ȼ�ͭ�ķ�������������(��ʵ���Ⱥ�˳������)��
�ٹ��� ����ȴ ������Ũ�� ����������
(4)Ϊ�˲ⶨ�Ƶõ��Ȼ�ͭ����(CuCl2��xH2O)��xֵ,ij��ȤС�����������ʵ�鷽��:
����һ:��ȡm g�����������������ټ���Ϊֹ,��ȴ������������ˮCuCl2������Ϊn g��
������:��ȡm g����,��������10%������������Һ�����ˡ�ϴ�Ӻ���С��������������ټ���Ϊֹ,��ȴ���������ù��������Ϊn g��
�ٷ���һ������CuCl2��xH2O�õ���ˮCuCl2,Ӧ����������(����������)�н��С�
������������һ����10%������������Һ,����������Ǽ��㡢����������������
����������������ʵ�鷽��,���п��еķ�������������,��һ�ַ��������е������� ��,���÷�����,�����x=��������������(�ú�m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����ij����(����Cu2O��Al2O3��Fe2O3��SiO2)��ȡͭ�IJ����������£�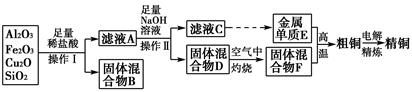
��֪��Cu2O��2H��=Cu��Cu2����H2O
(1)ʵ������������Ϊ________���ڿ��������չ�������Dʱ���õ����ֹ������ʵ������������������ƾ��ơ��������⣬����________(����������)��
(2)��ҺA����Ԫ�صĴ�����ʽΪ________(�����ӷ���)�����ɸ����ӵ����ӷ���ʽΪ____________________________________________��������ҺA�д��ڸ����ӵ��Լ�Ϊ________(���Լ�����)��
(3)��������E���������F������ijһ��Ӧ�����ں��Ӹֹ죬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
(4)�����£���pH��NaAlO2��NaOH������Һ�У���ˮ�������c(OH��)ǰ��Ϊ���ߵ�108������������Һ��pH��________��
(5)��Ũ���ᡢŨ���ᡢ����ˮ��ѡ�ú��ʵ��Լ����ⶨ��ͭ��Ʒ�н���ͭ�������������漰����Ҫ���裺��ȡһ����������Ʒ��________________�����ˡ�ϴ�ӡ����������ʣ�����ͭ��������(��ȱ�ٵIJ������裬���������������̵�ϸ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Co(OH)2�ڿ����м���ʱ��������������¶ȵı仯��ͼ��ʾ�� 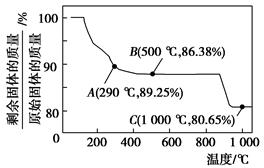
�ܵ��й��������£�Co(OH)2�������ԡ��ܵ���Ҫ��������ѧ�������������ӽ���������Ԫ�ء�
���������Ϣ�Իش��������⣺
(1)Co(OH)2���Ʊ�����CoCl2��Һ�м��Թ����İ�ˮ������NaOH��Һ��ԭ����________________________________(�û�ѧ����ʽ��ʾ)���Ƶõ�Co(OH)2�����ڿ����г��ڷ��ã��ᱻ�����е�O2��������ѧ����ʽΪ_____________________________________________________��
(2)��ͼ����֪�ܵ��������������290 ��ʱ����ȫ��ˮ����1000 ��ʱ��ʣ�����ijɷ�Ϊ________________________(�ѧʽ)����290��500 �淶Χ�ڣ������ķ�Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
(3)��֪�����£���0.10 mol��L��1 CoCl2��Һ�м��백ˮ��ֽ��裬��Co(OH)2�������ɣ�����Һ��pH��8ʱ��c(Co2��)��________mol��L��1(Ksp[Co(OH)2]��1.6��10��15)�������·�ӦCo2����2H2O??Co(OH)2��2H����ƽ�ⳣ��Ϊ
_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F ��������������ͼ��ʾת����ϵ����������D������Ϊ���壬����E�ǵ���ɫ��ĩ�������ַ�Ӧ������P��Ӧ��������ȥ��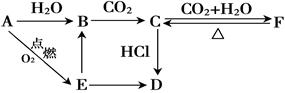
��1��д������A��D�Ļ�ѧʽ��A�� �� D�� ��
��2��д����������ת����Ӧ�����ӷ���ʽ��
B C�� ��
C�� ��
E B�� ��
B�� ��
C F�� ��
F�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��ӰĴ����ǽ��ڵ�ij��¯��������A��ʾ���ijɷ����£�������������
| C | Si | Mn | P | S |
| 4.070% | 2.900% | 0.495% | 0.165% | 0.010% |
| | FeO | Fe2O3 | CaO |
| �̳�����ǰ��%�� | 86.40 | 4.00 | 9.60 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com