
| 92g��cV��10-3mol |
| 2mol |
| 36cV��10-3 |
| a |
| 36cV��10-3 |
| a |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 36cV��10-3 |
| a |
| 36cV��10-3 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
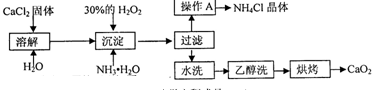
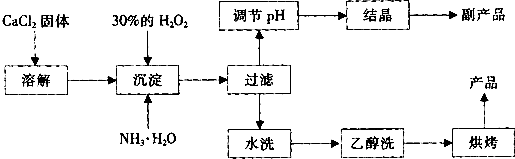
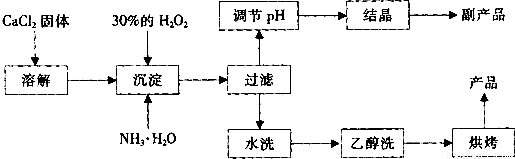
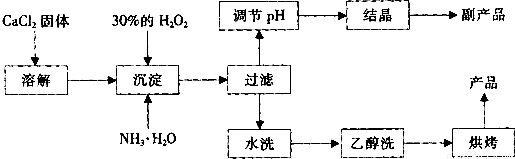
��12�֣������Ĺ������ƣ�CaO2���ǰ�ɫ�Ľᾧ��ĩ��������ˮ���������Ҵ������ѣ������½�Ϊ�ȶ�����һ������ˮ����ֳ���������������ʻ�ˮ��Ʒ�����䡣��֪��
![]() �� �ڳ�ʪ������CaO2�ܹ�������Ӧ��
�� �ڳ�ʪ������CaO2�ܹ�������Ӧ��
CaO2��2H2O �� Ca(OH)2��H2O2 2CaO2��2CO2 �� 2CaCO3��O2
![]() �� CaO2��ϡ�ᷴӦ�����κ�H2O2��CaO2��2H+ ��Ca2+��H2O2
�� CaO2��ϡ�ᷴӦ�����κ�H2O2��CaO2��2H+ ��Ca2+��H2O2
![]() ��ʵ���ҿ��ø�����ȡCaO2?8H2O���پ���ˮ�Ƶ�CaO2��CaO2?8H2O��0��ʱ�ȶ���������ʱ��������ͷֽ⣬������130��ʱ��Ϊ��ˮCaO2��
��ʵ���ҿ��ø�����ȡCaO2?8H2O���پ���ˮ�Ƶ�CaO2��CaO2?8H2O��0��ʱ�ȶ���������ʱ��������ͷֽ⣬������130��ʱ��Ϊ��ˮCaO2��
![]() ���Ʊ��������£�
���Ʊ��������£�

����������Ϣ���ش��������⣺w.w.w.k.s.5.u.c.
��1��������������ȡCaO2?8H2O�Ļ�ѧ����ʽ�� ��
��2��Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����� ��
��3�����Ʒ��ĸ���ƷΪ ���ѧʽ����Ϊ����߸���Ʒ�IJ��ʣ��ᾧǰҪ����Һ��pH���������ʷ�Χ���ɼ�����Լ��� ��
A������ B����ˮ C��ϡ���� D������������Һ
��4��Ϊ�˼��顰ˮϴ���Ƿ�ϸ�ķ�����
![]()
��5���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
![]() ��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��
Һ����ַ�Ӧ��
![]()
�ڶ�������������ƿ�м��뼸�ε�����Һ��
![]()
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
![]() ����֪��I2+2S2O32��= 2I��+S4O62������ɫ����
����֪��I2+2S2O32��= 2I��+S4O62������ɫ����
�ٵ�������˵����Ӧǡ����ȫ�������� ��
![]()
��CaO2����������Ϊ (����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2�������������� �������Ӱ�족����ƫ�͡���ƫ�ߡ�����ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010ѧ��㶫ʡ��ͷ�н�ƽ���߿���ѧģ���Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ���е������и߿���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com