
 ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 CH3OCH3(g) + CO2(g)����H��-247kJ/mol
CH3OCH3(g) + CO2(g)����H��-247kJ/mol| A�����¸�ѹ�� | B����������� | C�����������뺤���� | D������CO��Ũ�ȣ�E������������� |
 CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�
CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol��L��1�� | 0.01 | 0.2 | 0.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������



| ʵ���� | T(0C) | NO��ʼŨ�� ��mol/L�� | CO��ʼŨ�� ��mol/L�� | �����ı� �����(m2/g) |
| �� | 280 | 1��20��10-3 | 5��80��10-3 | 82 |
| �� | a | b | c | 124 |
| �� | 350 | d | e | 124 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 C(s)=
C(s)= CO2(g)��2Fe(s) ��H����234��1 kJ/mol
CO2(g)��2Fe(s) ��H����234��1 kJ/mol O2(g)=Fe2O3(s) �Ħ�H�ǣ� ��
O2(g)=Fe2O3(s) �Ħ�H�ǣ� ��| A����824��4 kJ/mol | B����627��6 kJ/mol |
| C����744��7 kJ/mol | D����169��4 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH��g������H=��90.8kJ��mol
CH3OH��g������H=��90.8kJ��mol
 CH3OCH3��g��+H2O��g������H=��23.5kJ��mol
CH3OCH3��g��+H2O��g������H=��23.5kJ��mol
 CO2��g��+H2��g������H=��41.3kJ��mol
CO2��g��+H2��g������H=��41.3kJ��mol
 CH3OCH3��g��+CO2��g���Ħ�H=__________
CH3OCH3��g��+CO2��g���Ħ�H=__________ H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��
H2��g��+CO2��g�����÷�Ӧƽ�ⳣ�����¶ȵı仯���±���ʾ��| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
 2NO2��g����H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǡ�
2NO2��g����H>0�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ���ǡ�
 CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
CH3OH(g)+H2O(g)��H=��49.0kJ��mol��1�������¶ȡ��ݻ���ͬ��3���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������¡�����˵����ȷ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


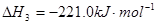
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0

 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 2CO2+ N2 ��H
2CO2+ N2 ��H


�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com