
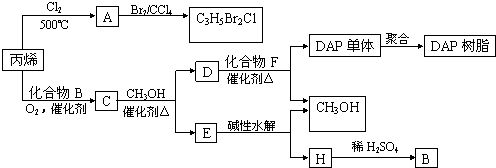
 ��������Ԫ�أ��ֱ�λ��Ԫ�����ڱ���ǰ�ĸ���ͬ���ڣ���ԭ�������ܺ�Ϊ48�������ǿ���ɼס��ҡ����������ֵ��ʺ�A��B��C��D���ֻ�������мס���Ϊ�ǽ������ʣ�������Ϊ�������ʣ���Щ���ʼ��ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ�
��������Ԫ�أ��ֱ�λ��Ԫ�����ڱ���ǰ�ĸ���ͬ���ڣ���ԭ�������ܺ�Ϊ48�������ǿ���ɼס��ҡ����������ֵ��ʺ�A��B��C��D���ֻ�������мס���Ϊ�ǽ������ʣ�������Ϊ�������ʣ���Щ���ʼ��ת����ϵ��ͼ��ʾ����Ӧ������ʡ�ԣ�| �� |
| ||
| ||
| ||
| ||
| �� |
| ���� |
| ���� |
| �� |
| ���� |
| ���� |
| �� |
| ||
| ||
| ||
| ||
| 21.6g |
| 72g/mol |
| 1 |
| 3 |
| 2 |
| 3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ���� |
| NaOH |
| �� |
| NaOH |
| �� |
| NaOH��Һ | ������Һ | ����Cu��OH��2 | ������ | |
| X | �кͷ�Ӧ | ������ | �� �� | �������� |
| Y | ������ | ������ | ���Ⱥ��к�ɫ���� | �������� |
| Z | ˮ�ⷴӦ | ������ | ���Ⱥ��к�ɫ���� | ������ |
| W | ˮ�ⷴӦ | ������ | ������ | ������ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� | CH4 | CH3CH3 | CH3��CH2��2CH3 | �������� |  |
 |
 |
| �е�/�� | -164 | -88.6 | -0.5 | �۵�/�� | 45 | 96 | 114 |
| 2 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ɽһ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ��ʴ���
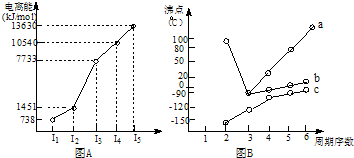
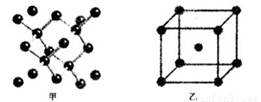
��18�֣�(1)I��������ij����Ԫ��M�ĵ������������ͼ(A)��ʾ����MԪ��λ�����ڱ��ĵ� �塣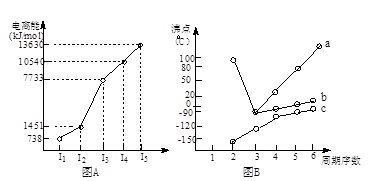
II��ͼB����c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ���λͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ�������������a��b������Ϊ��ȷ���ǣ�__________(�a����b��)
III�������л�����۷е���±���
| �� | CH4 | CH3CH3 | CH3(CH2)2CH3 | �������� | 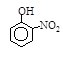 | 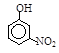 |  |
| �е�/�� | ��164 | ��88.6 | ��0.5 | �۵�/�� | 45 | 96 | 114 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�Ƹ��и���3������������ۻ�ѧ�Ծ��������棩 ���ͣ������
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ��ԭ�ӵļ۲�����������ڲ����������2����DԪ��ԭ�ӵ�L���Ӳ���ֻ�����ԳɶԵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3,��EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӡ���ش��������⣺
(1) EԪ�ػ�̬ԭ�ӵĺ���۲�����Ų�ʽΪ_________��
(2)��Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�����_________��
(3) AԪ����B��CԪ�ؿ��γɻ�����B2A4��C2A4��
��B2A4�ĽṹʽΪ_________��
�������й�C2A4��˵����ȷ����_________��
a.һ���÷����к���4���Ҽ�
b.�÷��ӿ���Ϊ��λ���γ���λ��
c.�÷����ǷǼ��Է��� d.1mol�÷��������γ�4mol���
e.�÷��ӵ��ȶ����������
f.�÷�����C��ԭ�ӹ����sp3�ӻ�
(4)B���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��E���ʵ�һ�ֵľ���ṹ��ͼ����ʾ��
��ͼ�еĵ���B������_________��ͼ����Bԭ�ӵ���λ����ͼ����Eԭ�ӵ���λ��֮_________��
EԪ����DԪ���γɵ�ED������NaCl����һ�������Ƚ�ED��NaCl�ľ����ܴ�С���迼�ǵ�������_______________________________________________________________________________��
(5)������������ʾ,F���ʵľ��������ж���,���侧���ֱ����������ܶѻ������������ѻ�����ʱ���䵥�ʵ��ܶ�֮��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��18�֣�(1)I��������ij����Ԫ��M�ĵ������������ͼ(A)��ʾ����MԪ��λ�����ڱ��ĵ� �塣
II��ͼB����c���Ա������ ��Ԫ���⻯��ķе�ı仯���ɡ���λͬѧ��ij����Ԫ���⻯��ķе�ı仯���ƻ�������������a��b������Ϊ��ȷ���ǣ�__________(�a����b��)
III�������л�����۷е���±���
|
�� |
CH4 |
CH3CH3 |
CH3(CH2)2CH3 |
�������� |
|
|
|
|
�е�/�� |
��164 |
��88.6 |
��0.5 |
�۵�/�� |
45 |
96 |
114 |
�������ʽṹ���ۣ�����Щ�������ܵó�����ؽ�����(����д2��)��
��
��
(2)COCl2�׳ƹ�����������Cԭ�Ӳ�ȡ �ӻ��ɼ�������̼��ԭ��֮�乲�ۼ�����
________(����ĸ)��
a��2���Ҽ���b��2���м���c��1���Ҽ���1���м���
(3)��������(22Ti) ���Ǽ�ͭ��������֮������㷺ʹ�õĵ����ֽ������Իش�
I��TiԪ�صĻ�̬ԭ�ӵļ۵��Ӳ��Ų�ʽΪ ��
II����֪Ti3�����γ���λ��Ϊ6������������ɫ����ɫ���ֺ��Ѿ��壬����ɾ�ΪTiCl3��6H2O��Ϊ�ⶨ�����־���Ļ�ѧʽ�����������ʵ�飺a���ֱ�ȡ�����������־������Ʒ�����Һ��b����������Һ�зֱ����AgNO3��Һ����������ɫ������c��������ȫ��ֱ���˵����ݳ�������ϴ�Ӹ����������ֲ����ij���������ϵΪ����ɫ����Ϊ��ɫ�����2/3������ɫ���������Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com