
��NA��ʾ�����ӵ�������ֵ��������������ȷ����(����)
A��1 mol BrCl��H2O��ȫ��Ӧ�����Ȼ���ʹ����ᣬת�Ƶĵ�����ΪNA
B��п��һ��Ũ�ȵ�Ũ���ᷴӦ��������״����SO2��H2�Ļ������22.4 L��пʧȥ������Ϊ2NA
C�����³�ѹ�£�20 g D2O���е�ԭ������Ϊ3NA
D����״���£�44.8 L NO��22.4 L O2��Ϻ������з�������С��2NA
���������⿼�鰢���ӵ����������ڿ��鿼�������ӵ����������⼰��ѧ����������BrCl��H2O�ķ�ӦΪ��BrCl��H2O===HBrO��HCl���˷�ӦΪ��������ԭ��Ӧ��A�������H2SO4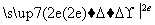 SO2��H2SO4
SO2��H2SO4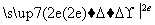 H2��Zn
H2��Zn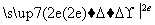 Zn2�����ɵ�ʧ�����غ�֪������1 mol����ʱ��пʧȥ������Ϊ2NA��B����ȷ��20 g D2O���е�ԭ������Ϊ
Zn2�����ɵ�ʧ�����غ�֪������1 mol����ʱ��пʧȥ������Ϊ2NA��B����ȷ��20 g D2O���е�ԭ������Ϊ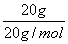 ��3��NA��3NA��C����ȷ����2NO��O2===2NO2֪����״���£�44.8 L NO��22.4 L O2��Ӧ����44.8 L NO2�����ڴ���2NO2===N2O4����˷�������С��2NA��D����ȷ��
��3��NA��3NA��C����ȷ����2NO��O2===2NO2֪����״���£�44.8 L NO��22.4 L O2��Ӧ����44.8 L NO2�����ڴ���2NO2===N2O4����˷�������С��2NA��D����ȷ��
�𰸣�A



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������������ȷ����_________������ţ���
A. ��ȡ��������ʱ����������Ҵ����μ��뵽Ũ������
B. ������Һ�м���(NH4)2SO4Ũ��Һ�������ʻ���������ˮ�����ܽ�
C. ���������ϩ�Ļ������ͨ��ʢ������������Ȼ�̼��Һ�У��ɳ�ȥ��ϩ
D. �ֱ�����������KMnO4��Һ��������Ȼ�̼��Һ�е��뱽��������ɫ��˵���������ڲ�����һ���̼̼˫��
E. ��ʵ������ȡ����ʱ��������������Եļ�飬�Ҹò���Ӧ��������װ�ú�װҩƷ֮ǰ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����е�һЩ���ⳣ�漰��ѧ֪ʶ��������������ȷ����(�����)
��
�ٵ��ۡ������ʶ�����Ȼ�л��߷��ӻ�����
��ʳ��ֲ���͵���Ҫ�ɷ��Ǹ�������֬������������������Ӫ������
�۽�ֹʹ�ú�Ǧ������Ϊ��������͵�ȼ��Ч��
��ϴ�Ӽ�ȥ���������仯����Һȥ�����ڻ�ѧ�仯
�����������������ļӿ죬�����С��װʳƷ�ѱ��㷺���ܡ�Ϊ�˷�ֹ�����±��ȸ�֬ʳƷ�������ʣ��ӳ�ʳƷ�ı����ڣ��ڰ�װ���г�������ʯ��
�������õ����ȼ����Ҫ�����ࣺһ����ѹ����Ȼ������һ��ΪҺ��ʯ���������Ƕ�����̼�⻯����
�߸���������һ�����õ�ɱ������Ҳ��������������ˮ
��Ϊ��֤��øϴ�·۵�ϴ��Ч����Ӧ�÷�ˮ�ܽ�ϴ�·�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪NA���������ӵ�������ֵ������˵����ȷ����(����)
A�����³�ѹ�£�8 g O2����4NA������
B��1 L 1 mol/L��H2SO4ˮ��Һ�к�����ԭ����Ϊ4NA
C����״���£�22.4 L O2��������ʱת�Ƶ�����һ��Ϊ4NA
D���ڱ�״���£�22.4 L��CH3CH2OH����NA������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Cu��Cu2O��CuO��ɵĻ�������100 mL 0.6 mol/L HNO3��Һ��ǡ��ʹ������ܽ⣬ͬʱ�ռ���224 mL NO����(��״��)������˵������ȷ����(����)
A������������ͭ�����ʵ���Ϊ0.025 mol
B�����������Cu�����ʵ���Ϊ0.005 mol��������Cu2O��CuO�����ʵ�����0.020 mol
C����������к�0.01 mol Cu��������Cu2O��CuO�����ʵ�����Ϊ0.005 mol
D���������Cu�����ʵ�����ȡֵ��ΧΪ0.005 mol<n(Cu)<0.015 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ӽ��ܵ����������ڰ뵼�幤ҵ�͵����մɵ�������һ�ִ��Ⱥܸߵ�������乤ҵ��ȡ�������£�
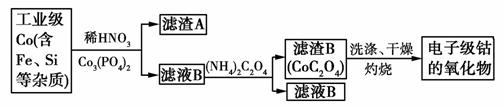
(1)ʵ���ҹ������������������̨����Ȧ���ձ���________��©����
(2)����A�ijɷֳ�������Co3(PO4)2��Fe(OH)3���________(�ѧʽ)������Co3(PO4)2��Ŀ����__________________��
(3)Co��ϡ���ᷴӦ����Co2�������ӷ���ʽΪ��_____________��
(4)����B��������ϴ�ӡ�����������գ������ط���ͼ���£�
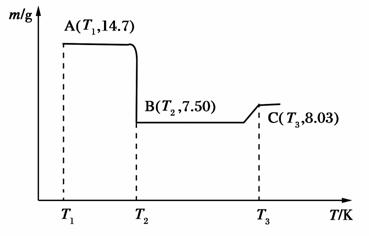
д�����л�ѧ��Ӧ����ʽ��
��AB��____________________________________________��
��BC��____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ԫ�������ɣ�±��Ԫ�ص��������ʴ��ϵ������εݼ�����
A���ǽ����� B��ԭ�Ӱ뾶
C�����ʵ������� D���⻯����ȶ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com