
| ŨH2SO4 |
| �� |

| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
 ��
�� ��
��| Ũ���� |
| �� |
| Ũ���� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Cu��OH��2����������Cu��OH��2��������
��Cu��OH��2����������Cu��OH��2��������| A��2.5mol | B��2mol |
| C��3mol | D��4mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��J��D��E��G��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������A����������Ԫ�ؼȲ���ͬһ���ڣ�Ҳ����ͬһ���壮J��Dͬ���壬E��Gͬ���ڣ�Ԫ��G�����ڱ��еĵ�7��Ԫ�أ�E�����������������ڲ��������ͬ��E��J���γ����ӻ�����侧���ṹ������Jԭ���ھ����ڲ�����ͼ��
��֪A��J��D��E��G��Ԫ�����ڱ���1��36��Ԫ�أ���ԭ��������������A����������Ԫ�ؼȲ���ͬһ���ڣ�Ҳ����ͬһ���壮J��Dͬ���壬E��Gͬ���ڣ�Ԫ��G�����ڱ��еĵ�7��Ԫ�أ�E�����������������ڲ��������ͬ��E��J���γ����ӻ�����侧���ṹ������Jԭ���ھ����ڲ�����ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� |
||||||||
| �� | ||||||||
| �� | ||||||||
| �� | �� | �� | �� | |||||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʵ�����Ʊ���ϩʱ�������Ƭ�����Է�ֹ���� |
| B����KMnO4�ζ�H2C2O4ʱ��Ҫ�õ���֧��ʽ�ζ��� |
| C�������к��ȵIJⶨʵ��ʱ�������õ�������Ͳ�������¶ȼ� |
| D��ʵ���Ҳⶨ��ѧ��Ӧ����ʱ����Ҫ�õ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
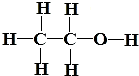
| A��CH3CH2OH��CH3OCH3��Ϊͬ���칹�� |
| B���ɱ��ͱ�Ϊͬһ������ |
| C��CH3CH3��CH3CH2CH3��Ϊͬϵ�� |
| D��O2��O3��Ϊͬ�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com