
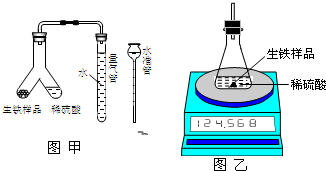
 Ϊ�ⶨij�������������Fe��C����ĩ״��Ʒ����������������ij��ѧ�о���ѧϰС������йط�����������ʵ�飮
Ϊ�ⶨij�������������Fe��C����ĩ״��Ʒ����������������ij��ѧ�о���ѧϰС������йط�����������ʵ�飮| ��Ӧǰ������װ��+ ϡ��������/g | ��Ӧǰ�� ������Ʒ����/g | ��Ӧ������װ��+ ��ƿ��ʣ���������/g |
| a | m | b |
| ʵ����� | �� | �� | �� |
| ����������Ʒ������/g | 1.43 | 2.86 | 8.58 |
| ������������/L����״���� | 0.56 | 1.12 | 2.24 |
| a+m-b |
| 2 |
| 56g/mol��(a+m-b) |
| 2mg |
| 28(a+m-b) |
| m |
| 28(a+m-b) |
| m |
| 5.72g-0.12g |
| 56g/mol |


 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ԭ������ | B���������� |
| C���������� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��MnO2��Ũ�����ϼ��ȣ�MnO2+4H++2Cl-
| ||||
| B�������ȥˮ����2H++CaCO3�TCa2++CO2��+H2O | ||||
| C����NH4��2Fe��SO4��2��Һ�����NaOH��Һ��Ӧ��NH4++OH-�TNH3?H2O | ||||
| D��Fe��ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
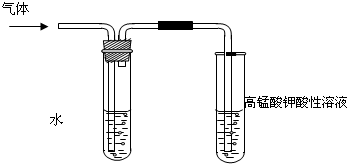 ѧϰ±����������ʱ������ʦͨ��ʵ��ķ�����֤�������ڲ�ͬ�ܼ�����NaOH ��Ӧ���ɲ�ͬ�ķ�Ӧ�������һ��������ǵ�̽����
ѧϰ±����������ʱ������ʦͨ��ʵ��ķ�����֤�������ڲ�ͬ�ܼ�����NaOH ��Ӧ���ɲ�ͬ�ķ�Ӧ�������һ��������ǵ�̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
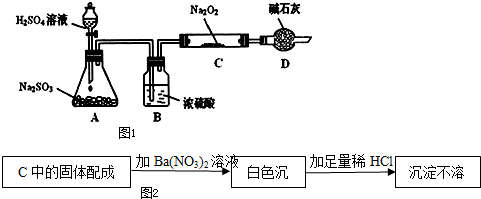
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��п�� | B��ͭ Ƭ |
| C����Ƭ | D��þ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʯ���ѽ����֬���������ɸ߷�������С���ӵĹ��� |
| B����ϩ����������ԭ�Ӳ�������ͬһƽ���� |
| C����CH3��3CCH2CH3��һ�ȴ�����3�� |
| D���ױ�������������������ˮ����ɿ���ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��
��1����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��| ��ѧ�� | C-H | Cl-Cl | C-Cl | H-Cl |
| ����/kJ?mol-1 | X | 243 | 330 | 432 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com