
�����е��л�������ḻ������ʳס�еȶ��Ӧ�ù㷺�������Ҵ��������DZȽϳ������л��
(1)��ҵ������ϩ��ˮ��Ӧ���Ƶ��Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪ________________(����д��Ӧ����)��ԭ����������__________��
(2)�Ҵ��ܹ�����������Ӧ��
�Ҵ���ͭ�������������¿ɱ���������Ϊ��ȩ����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
(3)���й����Ҵ���˵����ȷ����__________(ѡ����ĸ)��
A���Ҵ����ܺ����Ը��������Һ����������ԭ��Ӧ
B�������ó�ɫ�������ظ������Һ���˾���Ƿ�ƺ�ݳ�
C���ƾ���ijЩ����ʹ�Ҵ�����Ϊ���ᣬ���Ǿƾͱ�����
(4)����ʱ�ȼӴ��ּӾƿ������Ӳ˵���ζ����ԭ�����������������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ__________________________���÷�Ӧ�ķ�Ӧ����Ϊ______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��D�����л����һ��������������ת����ϵ��

��֪�ĵĻ�ѧʽΪC4H8O2����
��1��A���õĽṹ��ʽ���� �� ��
��2��д����ѧ����ʽ��C��D�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л�������к�һ��������һ������һ���ǻ�, �������ֽṹ�ķ������ʹ���
A��6�� B��5�� C��4�� D��3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ����ȡ����Ӧ���� (����)��
��CH3CH===CH2��Br2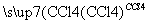 CH3CHBrCH2Br
CH3CHBrCH2Br
��CH3CH2OH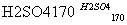 CH2===CH2����H2O
CH2===CH2����H2O
��CH3COOH��CH3CH2OH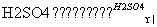 CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��C6H6��HNO3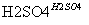 C
C 6H5NO2��H2O
6H5NO2��H2O
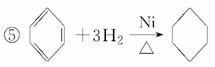
��2CH3CH2OH��O2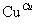 2CH3CHO��2H2O
2CH3CHO��2H2O
����ά������ˮ������������
����֬��������Ӧ
�ᰱ�������ɶ��ĵķ�Ӧ
��nCH2===CH2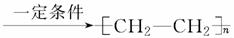
A���ۢܢߢ� B���٢ڢۢ�
B���٢ڢۢ�
C���ݢޢߢ� D���ۢܢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����������Һ�ܴ����������
A�� Mg2����K����SO42�� ��NO3�� B�� Na����OH����NH4+��HCO3��
C�� Al3+�� K���� SO32�� ��ClO�� D�� AlO2 -��H����Fe3����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����̼-��ż����Ӧ�ϳ����������л��ϳɵ��о��ȵ�֮һ���磺��Ӧ��

���������������ºϳ�·��ã�

��1���������ķ���ʽΪ_____________��1mol�û�������������ȫ�ӳ�ʱ��������ҪH2 mol��
��2����������������Ƶ�Cu(OH)2��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3�����������������NaOH��Һ��Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����������ǻ�������ͬ���칹��д����������������������Ľṹ��ʽ
��1mol���л�����������������Һ�ɵõ�4molAg
�������м��Եķ����廯����˴Ź��������еķ������Ϊ6:2:2:1��
��5���������ĺϳ�·���У�д���������Ľṹ��ʽΪ
 |
 ��6���л��� �� ��һ�������°����ʵ���2:1�ı�����������
��6���л��� �� ��һ�������°����ʵ���2:1�ı�����������
��Ӧ�ٵķ�Ӧ�����ɵ��л���������Ľṹ��ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йط������Ϸ��е�����������ȷ����
A.��Ҫ�����Ǹ߷��Ӳ��� B.���������ᡢ�������ܺ�
C.��������¯����ʳƷ D.������ʢ�ź��ͽ϶��ʳƷ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ij��ɫ��Һ���������ӵļ��������ж���ȷ���� (����)
A������AgNO3��Һ�����ɰ�ɫ��������ϡ����������ܽ�ʱ����ȷ����Cl������
B��ͨ��Cl2����Һ��Ϊ���ɫ�����������Һ����Һ��������ȷ����I������
C������Ba(NO3)2��Һ�����ɰ�ɫ��������ϡ�����������ܽ�ʱ����ȷ����SO ����
����
D������ϡ���ᣬ���ɵ�������ʹ����ʯ��ˮ����ǣ���ȷ����CO ����
����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com