
��11�֣�
��1����ˮ�е����Ρ�þ�εȶ�����Ҫ�Ļ���ԭ�ϣ��Ӻ�ˮ����ȡ�ˡ���ˮ��һ��������˵����ս�����塣
��д���Ȼ�þ�ĵ���ʽ__________________________
����ˮ��Ħ������Ϊ____________________________
��д������þ�Ĺ�ҵұ������ʽ__________________________________________
�ܺ�ˮ�����ij��������е������������ӽ�������_________________
��2�������У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ���������飨�е�12.27�棩���оֲ��䶳����Ӧ��������
����ϩ���Ȼ�����һ�������·�Ӧ�Ļ�ѧ����ʽ��____________________________
�ھ����������������䶳����Ӧ�������ľ���������________����ѡ�
| A���е�� | B���е�� | C���ӷ� | D���ѻӷ� |
��1����  ��1�֣�
��1�֣�
�� 20 g/mol ����λ��д�����֣� ��1�֣�
�� MgCl2�����ۣ� Mg+Cl2�� ��2�֣�
Mg+Cl2�� ��2�֣�
�� ���� ��1�֣�
��2�� �� CH2=CH2+HCl CH3CH2Cl ��2�֣�
CH3CH2Cl ��2�֣�
�� A C ��2�֣�
�� 5 ��1�֣�
��3�� CH4 ��д����Ҳ�ɣ� ��1�֣���
�������������
��1���� ����ʽD2O
�� ��������Ȼ�þ������ת��Ϊ��ѧ�ܡ� �����Ȼ�þ, �������״̬�µ��Ȼ�þ�������������ͽ���þ��
�� �е�ͣ�����ʱ�����մ������ȣ���ʹ���˲�λ���¶�Ѹ�ٽ��ͣ��Ӷ��ﵽ�ֲ��䶳������Ŀ�ġ�
�ۼ�����������Ӧ����һ�ȼ��飬���ȼ��飬���ȼ��飬���ȼ��飨�����Ȼ�̼�����Ȼ��⣬ֻ��һ�ȼ�����Ȼ�������̬����
���㣺��ˮ���ۺ����ã��绯ѧ����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij����Ԫ��R��������R(NO3)n��Һ��Pt�缫��⣬������������V L(��״��)ʱ��������������m g����R��ԭ����ΪM�����������в���ȷ���� (����)
A����·��ͨ������ mol mol | B��n= |
C����Һ��H������ mol mol | D��n= |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ͼ�У����缫�Ϸ����ĵ缫��ӦΪ��a����Cu2+ + 2 e��= Cu b����Fe - 2 e��= Fe2+
����˵���в���ȷ����
| A����װ�ÿ����ǵ��� |
| B��a����һ��������ԭ��Ӧ |
| C��a��b������ͬ�ֵ缫���� |
| D���ù�����������ת��һ���ǻ�ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ij����С������ͼ����ʵ�飬�Իش��������⡣
��1������ʼʱ����K��a���ӣ���B���ĵ缫��ӦʽΪ ��
��2������ʼʱ����K��b���ӣ���B���ĵ缫��ӦʽΪ ��
�ܷ�Ӧ�����ӷ���ʽΪ ��
��3��������K��b����ʱ������˵����ȷ����(�����) ��
����Һ��Na����B���ƶ�
�ڴ�A�����ݳ���������ʹʪ���KI������ֽ����
�۷�Ӧһ��ʱ����������������ɻָ������ǰ����ʵ�Ũ��
������״����B������2.24L���壬����Һ��ת����0.2mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣���1����п��ͭ�õ������Ӻ����2mol/L��ϡ�����У������缫��ӦʽΪ_________������ ��Ӧ����������ԭ�������Ӵ� �����·��____ ����п��ͭ������Һ��H+ �� �ƶ����������������ŵ�һ��ʱ���������pHֵ ������С�䣩��
��2����ͭƬ����Ƭ�õ������Ӻ�������Ȼ�����Һ�У�Ҳ�е���ͨ���������������缫��ӦʽΪ_________________���ܷ�Ӧ�����ӷ���ʽΪ______ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1991���ҹ��״���������������ˮΪ��Դ�����͡���ˮ��ء����õ���Ժ�ˮΪ�������Һ���������е���ʹ�������������������������ȸɵ��������20��50�������ܷ�Ӧʽ��ʾΪ��4Al��3O2��6H2O===4Al(OH)3��
��1���õ�Դ�ĸ�������Ϊ ����2��д��������ӦʽΪ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��U�ι���ʢ��100mL����Һ����Ҫ��ش��������⣺
��1����K2 ���ϲ�K1������ʢ��ҺΪCuSO4��Һ����AΪ ���� A���ĵ缫��ӦʽΪ ������ʢ��ҺΪKCl��Һ����B���ĵ缫��ӦʽΪ
��2����K1���ϲ�K2������ʢ��ҺΪ���з�̪��NaCl��Һ����
��A�缫�����ɹ۲쵽�������� ��Na+���� ������A��B��
��B�缫�ϵĵ缫��ӦʽΪ ��
�ܷ�Ӧ��ѧ����ʽ�� ��
�۷�Ӧһ��ʱ����K2,��������Һ������仯��������ܽ⣬B������������������״����Ϊ11.2mL������Һ��ֻ�ϣ���Һ��pHԼΪ ����Ҫʹ�������Һ�ָ���ԭ״̬������U�ι��ڼ����ͨ��һ������ ��[ pH=-lgc(H+) ]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣��Ȼ���ͭ��CuCl�����л��ϳɹ�ҵ��Ӧ�ýϹ㷺�Ĵ��������ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ�������������Ե�Ʒ�Һ����Ҫ��Cu2+��Fe3+�����Ʊ��Ȼ���ͭ�Ĺ����������£�

��ش��������⣺
��1������������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ��
��
��3������CuCl����ʱ�����pH�� ���ҡ�
��4��������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�Ȼ����ո����ȴ���ܷ��װ����ո���ܷ��װ��Ŀ���� ��
��5������ҺA�пɻ��յ���Ҫ������ ��д��ѧʽ����
��6����̼��Ϊ�缫���CuCl2��Һ�ɵõ�CuCl��д�����CuCl2��Һ��������Ϸ����ķ�ӦΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�׳ص��ܷ�ӦʽΪ�� 2CH3OH+3O2+4KOH 2K2CO3+6H2O
��1����ش�ͼ�мס������ص����ơ�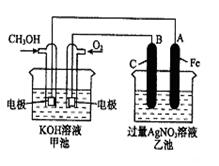
�׳��� װ�ã��ҳ��� װ�á�
��2����ش����е缫�����ƣ�ͨ��CH3OH�ĵ缫������ ��B��ʯī���缫�������� ��
��3��д���缫��Ӧʽ��ͨ��O2�ĵ缫�ĵ缫��Ӧʽ�� ��
��4���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��5�����ҳ���A��Fe��������������5��40gʱ���׳�������������O2 mL����״���£�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com