��2011?������ģ��[��ѧһѡ��3���ʽṹ������]
�±�Ϊ��ʽ���ڱ���һ���֣����е���ĸA--J�ֱ������Ӧ��10��Ԫ�أ�
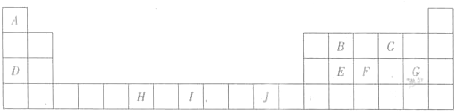
��ش��������⣺
��1��Ԫ��I��ԭ�ӽṹʾ��ͼΪ
��
��2��B��E��Ԫ�طֱ���Ԫ��C��ԭ�Ӹ�����Ϊ1��2�γɻ�����ʱ������ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ
sp
sp
��
sp3
sp3
�������ֻ�������۷е���ܴ��ԭ����
SiO2Ϊԭ�Ӿ��壬CO2Ϊ���Ӿ���
SiO2Ϊԭ�Ӿ��壬CO2Ϊ���Ӿ���
��
��3��H
3+������һ�����������γɽṹ���ӵ�������[HG��A
2C��
5]
2+�γɸ�������ʱ��H
3+���ӽ����������ṩ��
�µ��Ӷ�
�µ��Ӷ�
�����������к��еĻ�ѧ��������
���ۼ������Ӽ�
���ۼ������Ӽ�
������G�ĺ�������Ų�ʽΪ
1s22s22p63s23p5
1s22s22p63s23p5
��
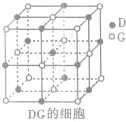
��4��Ԫ��D��Ԫ��G�γɵĻ�����DG�ľ����ṹ��ͼ��ʾ��ÿ��D������Χ��֮�����D���ӵĸ���Ϊ
12
12
������þ������ⳤΪa cm�������ӵ�������ֵΪN
A����û�������ܶ�Ϊ
��


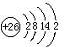
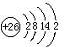
 ��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��
��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��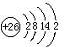 ��
��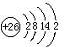 ��
��

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�




 +��n-1��H2O
+��n-1��H2O
 +��n-1��H2O
+��n-1��H2O ��
�� ��
��
 ��
�� ��
��

