
�������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2
�ܷ������·�Ӧ��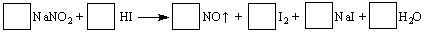
��1����ƽ������Ӧ����ʽ����ϵ�����뷽���С�
��2��������Ӧ���������� ������Ӧ����5 mol����ת�ƣ�������NO�ڱ�״���µ������ L��
��3������������Ӧ��������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У���ˮ���ڵ⻯�ص�����ֽ���۵��ۣ��ܰƣ���ʳ�ף�����ʵ�飬���м�����ʵ��� ��
| A���ۢ� | B���٢ڢ� | C���٢ڢ� | D���٢ڢۢ� |
��1��2NaNO2 + 4HI ��2NO + I2 +2 NaI +2 H2O ��2��NaNO2��112 ��3��C
���������������1�����ݵ�ʧ�����غ㷨��ƽ����ʽ������������NΪ+3�ۣ���Ӧ��Ϊ+2�ۣ�HI��IΪ-1�ۣ���Ӧ���Ϊ��������Ϊ0�ۣ����Դ�Ϊ2NaNO2 + 4HI ��2NO + I2 +2 NaI +2 H2O��
��2����������Ԫ�ػ��ϼ۽��͵����ʣ�������������NaNO2��ÿ����1molNO,��ת��1mol���ӣ�����Ӧ����5 mol����ת�ƣ�������5molNO,��״���µ������112L��
��3�����ݣ�2����֪���������������������£���������������Ϊ�ⵥ�ʣ��������۱���ɫ������ѡ���һ���Լ���C��
���㣺����������ԭ��Ӧ����ʽ����ƽ�����㣬���������жϣ����ʵļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ѧ���ճ������о��й㷺��Ӧ�ã�����Ӧ���в��漰������ԭ��Ӧԭ������
| A��������[KAl(SO4)2��12H2O]��ˮ | B����ˮ����ȡ�� |
| C��������CrO3����˾���Ƿ�ƺ��ʻ | D��ҽ������˫��ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�Q��W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������Wԭ������������������������֮��Ϊ3��4��Q��Y���������ǵ����������Ҫ���ʣ�X�ǵؿ��к�����ߵĽ���Ԫ�أ�Z���γɺ�ɫ����ש��ɫ����Z2O�ͺ�ɫ��ZO�������������������л�ѧ����ش� ��1��Q���ʵĵ���ʽΪ_______��
��1��Q���ʵĵ���ʽΪ_______�� W��X��Y�����Ӱ뾶�ɴ�С��˳��Ϊ____�������ӷ��Żش𣩡�
W��X��Y�����Ӱ뾶�ɴ�С��˳��Ϊ____�������ӷ��Żش𣩡� ��2��X��Y��ɵĻ��������ˮ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
��2��X��Y��ɵĻ��������ˮ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
��3��Na2Y��Һ������Ũ���ɴ�С��˳��Ϊ____________________________________________�� ��4��ZO�ڸ����±�Q�ļ���̬�⻯�ﻹԭΪZ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________��
��4��ZO�ڸ����±�Q�ļ���̬�⻯�ﻹԭΪZ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________��
��5��ZCl2��Һ�л���FeCl3����ʱ���ɼ���_____________�����Լ�������pH��_________���ٹ��ˡ���֪��Fe(OH)3��Ksp��10-35����ѧ����Ϊ��������Һ�е�����Ũ��С��1��10-5mol/Lʱ�������ʹ���ȫ��
��6����ҵ�Ͽ��ø���������Z2Y + O2��2Z + YO2��ұ������Z������1molZʱת��____mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(16��) �Ե�ⷨ���������̲������������У����̺����ߴ�40%��50%����Ҫ�ɷ�ΪMnO2��ˮ����Һ�����������Pb2+��Sn2+���ؽ������ʡ������ղ��õ�ľм��������ά���ڽ�Ũ��������������͡�ˮ�����ɻ�ԭ�ǣ����������£�����������̷�Ӧ���ɿ����Ե������̡�

��1����ҵ�ϵ�������̵�ˮ��Һ���������̣������ĵ缫��ӦʽΪ ��
��2��д��ľмˮ�ⷴӦ�Ļ�ѧ����ʽ�� ����ƽ�������跴Ӧ����ʽC6H12O6 + MnO2 + H2SO4�� MnSO4 + CO2 + H2O
��3����ȡ������������裬������� ���壨�ѧʽ������Ⱦ������
��4�������յ�һ�ָ���Ʒ����Ҫ��ũҵ�������ϣ�д���仯ѧʽ ��
��5��ȡһ���������������ʵ��������õ�����ͼ����������������Ϊ mL������������������������ᵼ�� ���ѧʽ��������������
��6��ij��������MnO2����Է�������Ϊ87������������Ϊ50.0%����174g����������320g36.5%��Ũ�����ϼ��ȣ�������������ڱ�״����Ӧ���� L���������������е������ɷֲ����뷴Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ����ͭ���õĺ�ɫ���������ͭ��������ͭ�Ļ�����֪Cu2O��������Һ�пɷ�������������ԭ��Ӧ������Cu2+��Cu��
��1������8.4������ͭ��������ȫ��ԭ�õ���ɫ����6.96�ˣ����к�����ͭ��������ͭ�����ʵ���֮���� ��
��2������6.96�������������������Ũ�����ַ�Ӧ��
�����ɱ�״����1.568�������壨������NO2���ܽ⣬Ҳ������NO2��N2O4��ת�������������ijɷ��� �������ʵ���֮���� ��
�ڰѵõ�����ҺС������Ũ�����������ľ�����ˣ��þ���20.328g����������ԭ��Һ�е�Cu2+��20%������ĸҺ�У����þ���Ļ�ѧʽΪ ��
��3��Cu��Cu2O��CuO��ɵĻ�������100mL 0.6mol/L HNO3��Һǡ��ʹ�������ȫ�ܽ⣬ͬʱ�ռ���224mL NO����(��״��)����ԭ�������Cu�����ʵ���ΪX��������Cu2O��CuO�����ʵ�����X��ȡֵ��Χ(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û��ϼۺ���������Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�
��1���ӻ��ϼ۵ĽǶȿ���Ԥ�����ʵ����ʡ�
�ٽ� ͨ������
ͨ������ ��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
��Һ�У���Һ����ɫ������ɫ����Ӧ��������Ԫ�ش�����ʽ�������� ��
| A��S2- | B��S | C��SO32- | D��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��10��������̨��������ܵ��ش���ʧ���м������Ľ����ɹ�����ҩƷ���������ֺ���Ҫ�����������������������и�Ч���������̼�����������Ҫ�ɷ�H2O2��һ����ɫճ��Һ�壬��ش��������⣺
��1�����з�����H2O2�����ֵ��������������Ϊ��������ȫһ�µ��� ��
A��BaO2+2HCl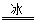 H2O2+BaCl2
H2O2+BaCl2
B��Ag2O+H2O2 =2Ag+O2+H2O
C��2H2O2 2H2O+O2��
2H2O+O2��
D��H2O2+NaCrO2+NaOH=Na2CrO4 +H2O
��2��������䳣��Һ̬��(N2H4)Ϊȼ�ϣ�Һ̬H2O2Ϊ��ȼ������֪��
N2H4��1��+O2(g)=N2(g)+2H2O(g) ��H=" -" 534 kJ��mol��1
H2O2��1��=H2O��1��+1/2O2(g) ��H=" -" 98.64 kJ��mol��1
H2O��1��=H2O(g) ��H=+44kJ��mol��l
��ӦN2H4��1��+2H2O2��1��=N2(g)+4H2O(g)�ġ�H= ��
�÷�Ӧ�ġ�S= 0(�������<��)��
��3��H2O2��һ�ֲ��ȶ��ֽ�����ʡ�
����ͼ��H2O2��û�д���ʱ��Ӧ�����������仯ͼ������ͼ�ϻ���ʹ�ô����ӿ�ֽ�����ʱ���������ͼ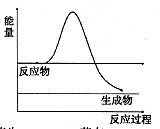
��ʵ��֤ʵ����Na2CO3��Һ�м���H2O2Ҳ�������ݲ�������֪����ʱH2CO3�ĵ��볣���ֱ�ΪKal=4.3��l0��7��Ka2 =" 5.0" ��l0��11 ��Na2CO3��Һ��CO32����һ��ˮ�ⳣ������ʽKhl= ������ʱKhl��ֵΪ ������Na2CO3��Һ��ͬʱ��������Na2CO3�������ʵ�������Һ�¶ȣ���Khl��ֵ
(����С�������ȷ��)��
��4��ij���ױ����˲�ͬ�������Ӽ���Ũ�ȶ�˫��ˮ�������⺣��������Һ��Ӧ���ʵ�Ӱ�죬ʵ������ͼ1��ͼ2��ʾ��
ע������ʵ������¶�Ϊ20�桢w(H2O2)=0��25%��pH=7��12������������ҺŨ��Ϊ8mg��L-l�������½��С�ͼ1������a��H2O2��b��H2O2+Cu2+��c��H2O2+Fe2+��d��H2O2+Zn2+��e��H2O2+Mn2+��ͼ2������f����Ӧʱ��Ϊ1h��g����Ӧʱ��Ϊ2h����ͼ�е��������������������Һ��ճ��(��������Ũ������Һճ�������)��
��������Ϣ��֪����������������� (�����)��
A����������ʹ�ý��ⷴӦ���ʼ���
B���������ӶԸý��ⷴӦ�Ĵ�Ч�ʱ�ͭ���ӵ�
C������������Һճ�ȵı仯�����ɷ�ӳ���併�ⷴӦ���ʵĿ���
D��һ�������£�ͭ����Ũ��һ��ʱ����Ӧʱ��Խ��������������ҺŨ��ԽС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ������Ƿۡ�����غ�ľ̿��һ��������϶��ɵģ���ըʱ�����N2��CO2�ȡ�
��1����ըʱ�Ļ�ѧ����ʽΪ�� ����2�֣�
��2���ںڻ�ҩ��ը�ķ�Ӧ����������������һЩ��Ӧ����Ҳ��������ԭ����д��һ�����ڷ�Ӧ������ԭ���Ļ�ѧ����ʽ�� ����2�֣�
��3�������ڷŵ���������������Ӧ�Ļ�ѧ����ʽ�� ����2�֣�
��4��һ�������������������ǵ������һ�������ڳ����º�����������е��������ϣ���ѧ����ʽΪ�� ����2�֣�������������ˮ��Ӧ����ѧ����ʽΪ�� ����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
(1)����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)��10C(s)=6CaO(s)��P4(s)��10CO(g)�� ��H1����3359.26 kJ��mol��1
CaO(s)��SiO2(s)=CaSiO3(s) ��H1����89.61 kJ��mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)=6CaSiO3(s)��P4(s)��10CO(g)�� ��H3
��H3��________kJ��mol��1��
(2)�����ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P4��60CuSO4��96H2O=20Cu3P��24H3PO4��60H2SO4
60 mol CuSO4�������������ʵ�����________��
(3)����Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ����ͼ��ʾ��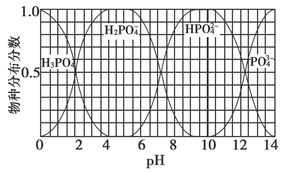
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������________��pH��8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ________��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����________(�����ӷ���ʽ��ʾ)��
��4���Ļ������������ף� ���뼾���Ĵ���
���뼾���Ĵ���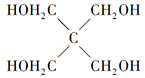 �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��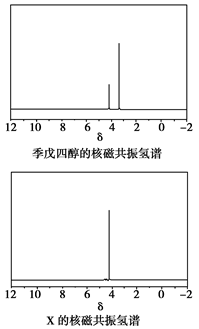
������������______________________(�ѧʽ)��
��X�Ľṹ��ʽΪ__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com